Gd2O3-rich borogermanate scintillation glass, and preparation method and application thereof
A technology of scintillation glass and borogermanic acid, applied in scintillation glass materials and its preparation, and in the field of Gd2O3 boorgermanate scintillation glass and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0066] 1. The preparation process of scintillation glass is simple, the chemical composition is easy to adjust, easy to realize large size, good chemical stability, and can be further drawn into optical fiber;
[0067] 2. Scintillation glass is rich in Gd 2 o 3 , on the one hand can effectively sensitize Ce 3+ , Tb 3+ and Eu 3+ Rare earth ions or Mn 2+ 、 Bi 3+ The luminous efficiency of transition metal ions greatly improves the output of scintillation light; on the other hand, it can greatly improve the density of scintillation glass (glass density up to 5.7g / cm 3 ), so that it meets the requirements of practical applications;
[0068] 3. The types of luminescent centers in the scintillation glass and their doping amount can be selected freely, which can effectively control the emission wavelength and decay time of the scintillation glass to meet the needs of practical applications.
Embodiment 1
[0072] 1. Preparation process
[0073] The first step: the glass formula is 25B 2 o 3 -50 GeO 2 –24Gd 2 o 3 –1CeO 2
[0074] Step 2: After fully mixing the components, melt them in an air atmosphere at 1450°C for 3 hours;
[0075] Step 3: Pour the above melt into a preheated 400°C stainless steel mold for casting, and cool naturally to form glass;
[0076] Step 4: Place the above glass in a muffle furnace at 550°C for 10 hours for annealing treatment;
[0077] Step 5: After cutting, surface grinding and polishing, the above-mentioned preliminary scintillating glass is processed into scintillating glass of 15×15×2 mm.
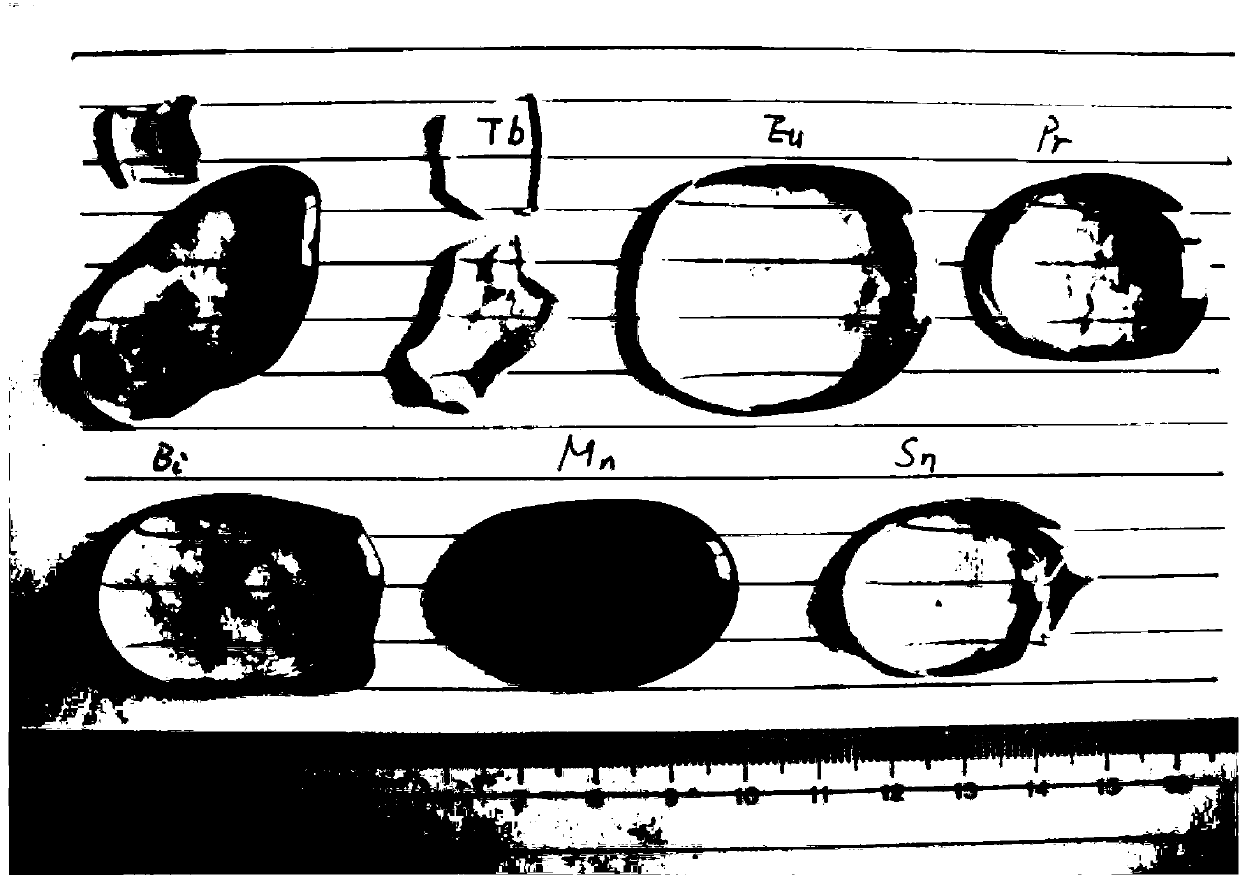
[0078] The photo of the prepared scintillation glass (before cutting and polishing) is shown in figure 1 shown.
[0079] 2. Test
[0080] The photoluminescence spectrum and X-ray excitation of the scintillation glass were tested with a Hitachi fluorescence spectrometer (Hitachi F-7000, Ex slit 5nm, Em slit 2.5nm) and an X-ray excitation emission spect...
Embodiment 2
[0084] Substantially the same as Example 1, the only difference is that the glass component is: 40B 2 o 3 –30GeO 2 –29Gd 2 o 3 –1Tb 2 o 3 , the luminescent center becomes Tb 3+ Ions, the melting atmosphere is air.
[0085] The photo of the prepared scintillation glass is as figure 1 shown.
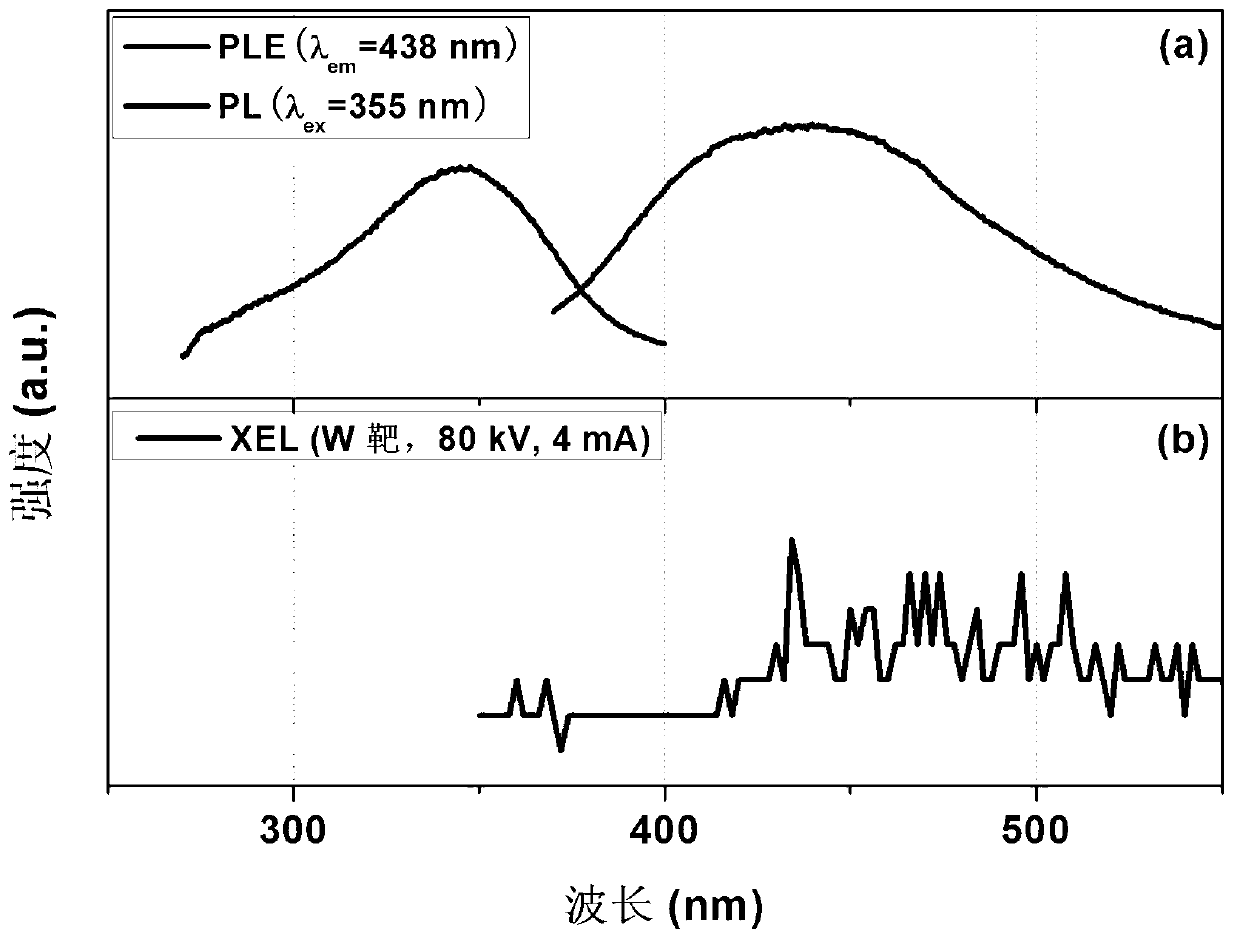
[0086] Using a fluorescence spectrometer (Hitachi F-7000, Ex slit 5nm, Em slit 2.5nm) and an X-ray excitation emission spectrometer (designed by ourselves, W target, 80kV, 4mA) to test the photoluminescence spectrum and X-ray excitation emission of the scintillation glass Spectrum, such as Figure 4 shown. from Figure 4 It can be seen that there are four luminescence peaks at 498nm, 542nm, 583nm and 620nm, corresponding to Tb 3+ ion 5 D. 4 → 7 f J (J=6, 5, 4, 3) optical transition, where 542nm ( 5 D. 4 → 7 f 5 ) wavelength has the highest scintillation luminescence peak intensity, and has a larger scintillation light output; at the same time, Gd 3+ Can effectively sen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com