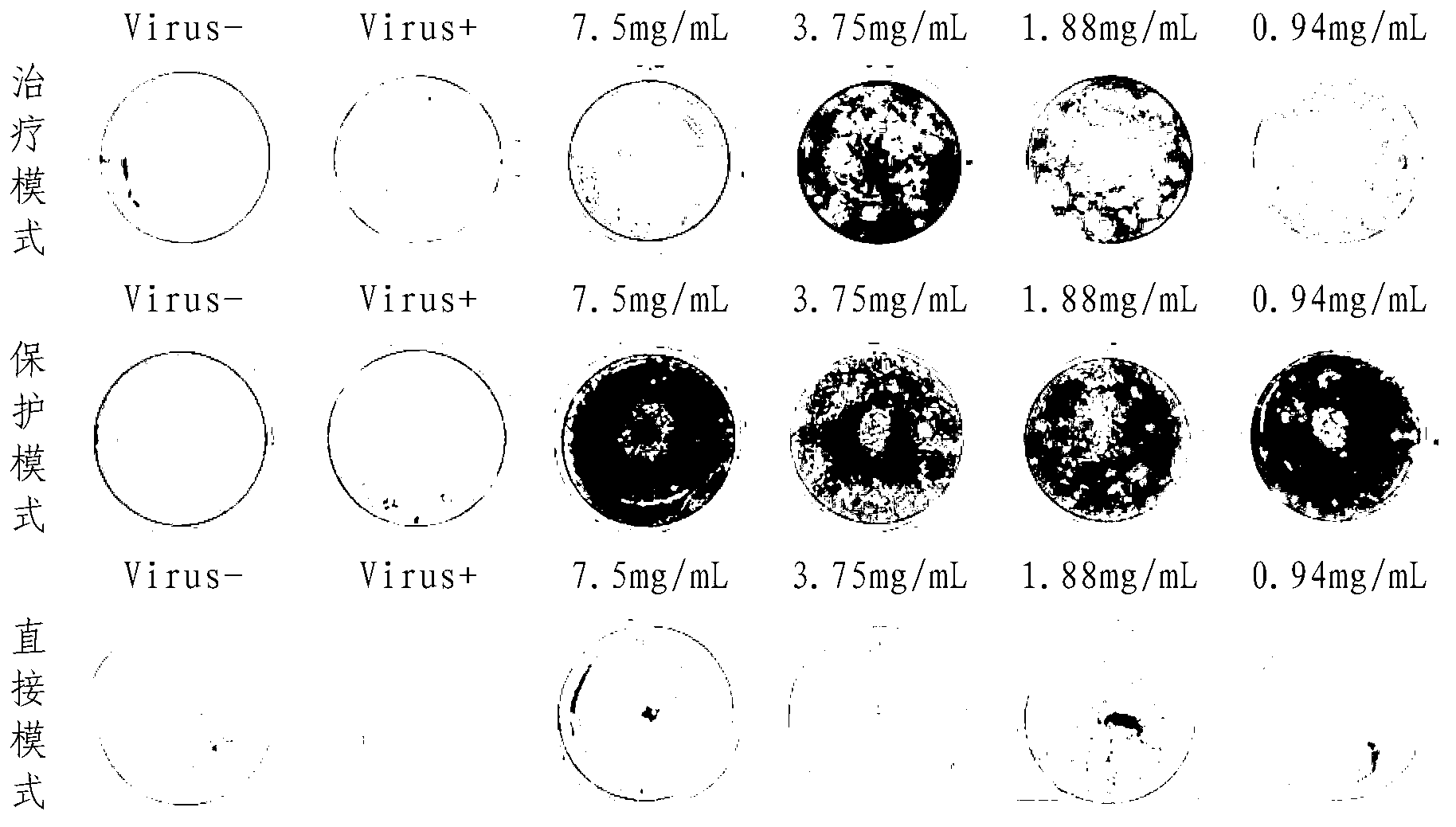Application of indole-3-acetonitrile-6-O-beta-D-pyran glucoside in pharmacy
A technology of glucopyranoside and acetonitrile, which is applied in the field of medicine, can solve the problems such as unclear anti-influenza virus components, and achieve the effects of less drug resistance, less cytotoxic effect, and convenient administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Preparation of indole-3-acetonitrile-6-O-β-D-glucopyranoside
[0035] A. Get 10 kg of Radix Radix Radix crude drug, chop it up, add 100 L of water to reflux and extract three times, each time for 2 hours, combine the extracts, concentrate under reduced pressure to a certain volume;
[0036] B. Place in D101 macroporous resin, elute with water first, then elute with 30% (V / V) ethanol, collect the eluent, concentrate under reduced pressure to obtain extract 1;
[0037]C. After the extract is dissolved in an appropriate amount of water, put it in an MCI gel column, elute with water, 25% (V / V) methanol, and 35% (V / V) methanol respectively, and collect 35% (V / V) V) methanol eluate, concentrated to dryness, to obtain extract 2
[0038] D. Extraction 2 is placed in a silica gel column, and is eluted with chloroform-methanol with a volume ratio of 20:1 and 10:1 respectively, wherein the eluted part of 10:1 chloroform-methanol obtains a brownish-yellow needle crystal, and chlor...
Embodiment 2
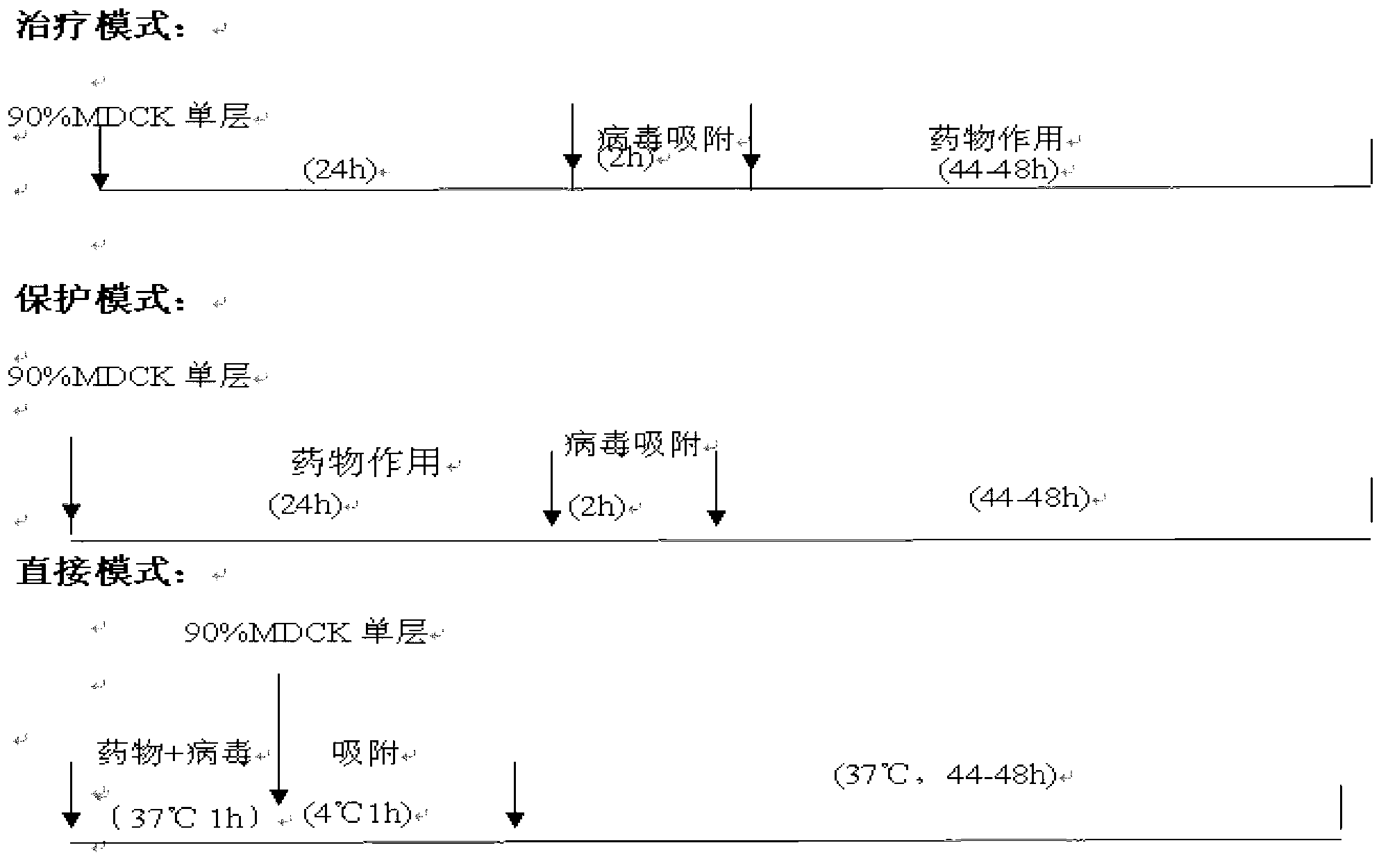
[0041] Anti-influenza virus effect of indole-3-acetonitrile-6-O-β-D-glucopyranoside in vitro
[0042] 1 Experimental materials
[0043] 1.1 Drug Test sample:
[0044] Indole-3-acetonitrile-6-O-β-D-glucopyranoside is self-made in the laboratory (the preparation method is as described in Example 1), and its structure is determined to be indole Indole-3-acetonitrile-6-O-β-D-glucopyranoside (Indole-3-acetonitrile-6-O-β-D-glucopyranoside, its purity was 98% by HPLC). Indole-3-acetonitrile-6-O-β-D-glucopyranoside is also available commercially.
[0045] 1.2 cells
[0046] Dog kidney cells (MDCK) were quoted from the Cell Bank of the Type Culture Collection Committee of the Chinese Academy of Sciences. Human laryngeal carcinoma cells (HEP-2), monkey kidney cells (LLC-MK2), and human embryonic lung fibroblasts (MRC-5) were cited from the American Classic Culture Collection (ATCC) and the Chinese Academy of Sciences Type Culture Collection Committee, respectively. cell bank.
[0...
Embodiment 3
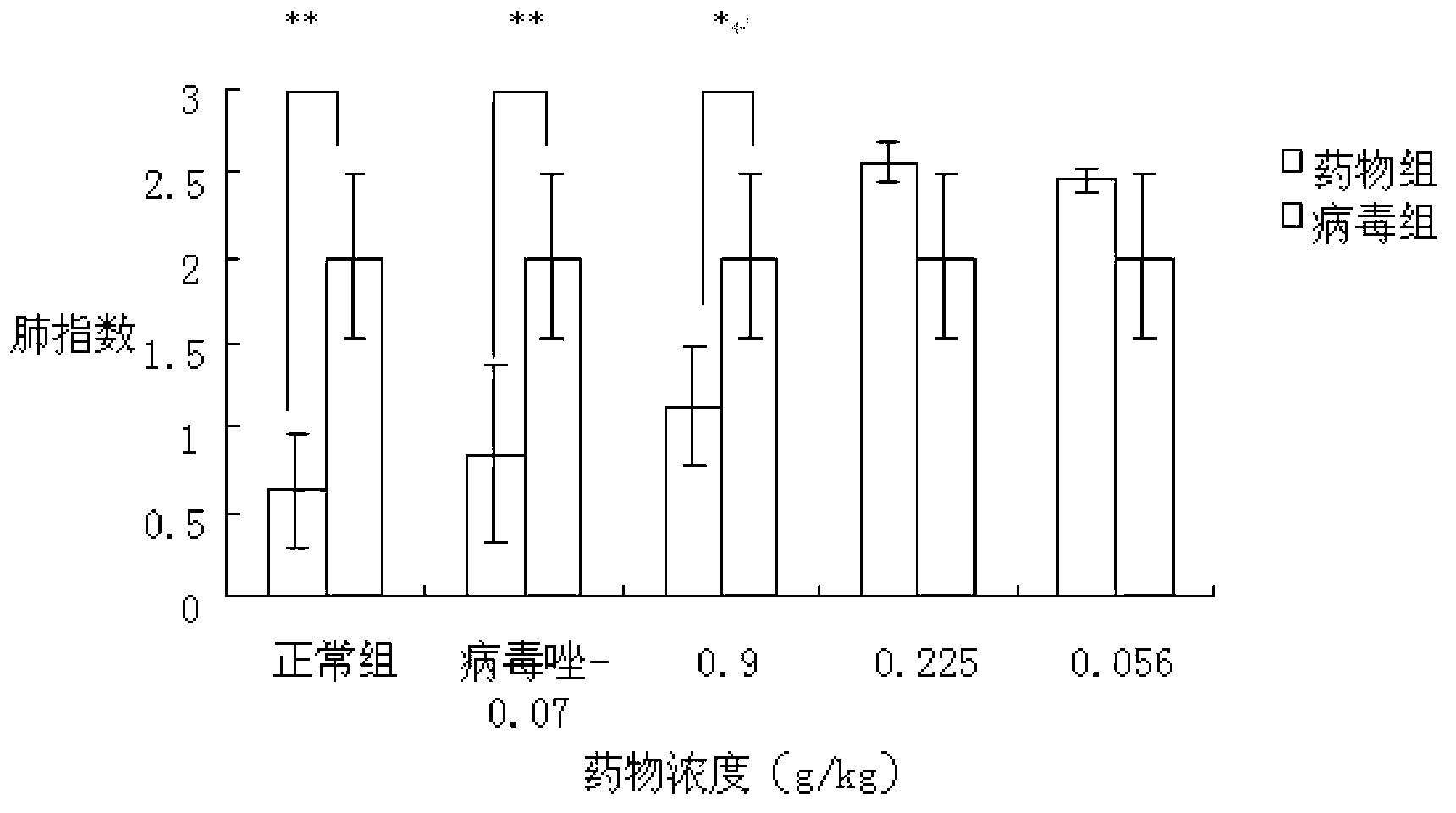
[0085] Inhibitory effect of indole-3-acetonitrile-6-O-β-D-glucopyranoside on influenza virus in vivo
[0086] 1. Experimental materials
[0087] 1.1 Drugs and their preparation
[0088] The positive control drug ribavirin (ribavirin) was purchased from Guangdong Zhaoqing Xinghu Biotechnology Co., Ltd.
[0089] 1.2 Animals
[0090] BALB / C mice (SPF grade), 6-8 weeks old, weighing 16-18g, were purchased from Guangdong Experimental Animal Center (permit number: SCXK (Guangdong) 2003-0002, Guangdong Jianzhengzi 2008A023).
[0091] 1.3 Virus strains
[0092] Mouse lung-adapted strain of influenza A (H1N1) virus (A / FM1 / 47, H1N1). During the expansion, the allantoic cavity of 9-day-old chicken embryos was inoculated, cultured at 35°C for 2 days, and the allantoic fluid was collected for later use. Determination of virus titer: the hemagglutination titer is 1024; according to the method of Reed-Muench [1] Determine the median lethal dose (LD 50 ) for 10 -3.5737 ; The plaque-fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com