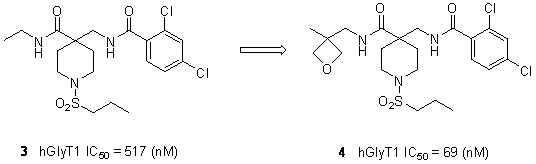
Preparation method of 3-aminomethyl oxetane and its organic acid salts
A technology of aminomethyl oxetane and oxetane, which is applied in the field of preparation of 3-aminomethyl oxetane and organic acid salts thereof, and can solve the problems of unsuitable amplification and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 : Preparation of 3-(nitromethane)oxetan-3-ol
[0033]
[0034] Operation steps: In a 250 ml single-necked bottle, add nitromethane (36 ml) and 3-oxetanone 1 (3.7 g, 51.3 mmol), triethylamine (70 mg) was added under stirring, and reacted at room temperature for 10 hours. The reaction solution was rotary evaporated in vacuo to remove excess nitromethane to obtain 6.8 grams of light yellow liquid 3-(nitromethane)oxetan-3-ol 2 , the yield is 100%.
[0035] HNMR (CDCl 3 ) δ4.83 (s, 2H), 4.72 (d, J = 8.0 Hz, 2H), 4.60 (d, J = 8.0 Hz, 2H).
Embodiment 2
[0036] Example 2 : Preparation of 3-(nitromethylene)oxetane
[0037]
[0038] In a 1-liter three-neck flask, add dichloromethane (300 ml) and 3-(nitromethane)oxetan-3-ol (6.8 g, 51.3 mmol), and cool to –78°C with a dry ice acetone bath . Triethylamine (15.6 g, 154 mmol) was then added dropwise, followed by the slow addition of methanesulfonyl chloride (11.8 g, 104 mmol) in dichloromethane (80 ml) dropwise over 3 hours. After the addition was complete, the reaction was continued to stir at -78°C for 2 hours, then returned to room temperature and stirred for 12 hours. The reaction solution was quenched by pouring into ice water, and extracted with dichloromethane. The organic phase was washed with saturated brine, dried over anhydrous sodium sulfate and filtered. After distilling off the solvent under reduced pressure, the filtrate was purified by column chromatography. The eluent is petroleum ether: ethyl acetate = 15:1. Finally, 3.8 g of white solid product was obtai...
Embodiment 3
[0040] Example 3 : Preparation of 3-Aminomethyloxetane Oxalate (Palladium Hydroxide on Carbon and Methanol, Room Temperature, 1 Atm of Hydrogen)
[0041]
[0042] Operation steps: 3-(nitromethylene)oxetane 3 (0.88 g, 7.7 mmol) and 0.3 g of palladium hydroxide on carbon (10% by mass) were added to the reaction flask, 20 ml of methanol was added, stirred at room temperature under 1 atmosphere of hydrogen for 48 hours, and then filtered. The filter cake was thoroughly washed with methanol, and the resulting filtrate was evaporated to remove the solvent under reduced pressure to obtain 1.2 g of a colorless liquid (the solvent methanol remained).
[0043] HNMR (MeOD) δ4.80 (t, J= 6.4 Hz, 2H), 4.40 (t, J= 6 Hz, 2H), 3.05 (m, 1H), 2.97 (t, J= 7.2 Hz, 2H).
[0044] Dissolve the above liquid in 10 ml of tert-butyl methyl ether, then add anhydrous oxalic acid (0.34 g, 3.8 mmol) solution dissolved in about 2 ml of ethanol to the above solution, immediately a white solid precipitate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com