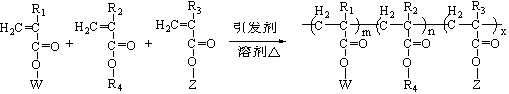
Imitation mussel attachment protein and cell membrane structure copolymer and preparation method and application thereof
A mussel adhesion protein and adhesion protein technology, applied in the fields of polymer chemistry and physics, surface science and biomedical materials, can solve the problem of unstable physical adsorption polymer coating, unsatisfactory modification effect, coating process Complicated problems, to achieve the effect of improved surface hydrophilicity and biocompatibility, good biocompatibility, and simple construction method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
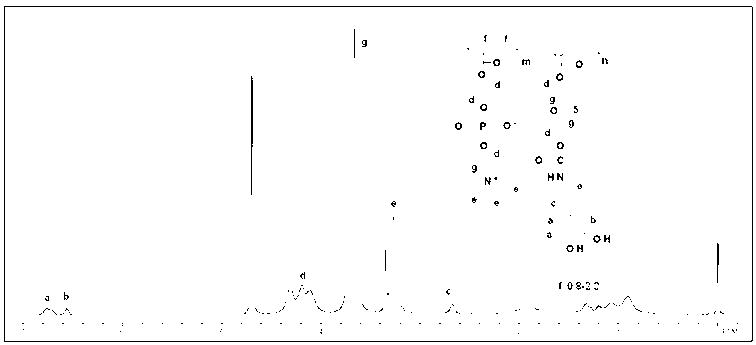
[0041] The preparation of the double biomimetic polymer PMCA: under the condition of passing nitrogen, the PMPA copolymer is mixed with 1.5 times equivalent of dopamine hydrochloride or dopa and triethylamine (diisopropylethylamine) in anhydrous organic solvent, React in a reaction flask at 50-78°C for 12 hours. The product solution was dialyzed in an aqueous solution of hydrochloric acid with a pH of 3.0-4.0 to remove impurities, and freeze-dried to obtain a dopamine-imitated cell membrane structure copolymer, ie, a double biomimetic copolymer PMCA. The content of hydrophilic units and catechol groups in the double biomimetic copolymer can be controlled by 1 Confirmed by H NMR characterization.
[0042] The above-mentioned double biomimetic copolymer PMCA can be used to prepare a membrane structure coating imitating the outer layer of cells. The following method is used to prepare the imitation cell outer layer membrane structure coating with the double biomimetic copolymer...
Embodiment 1
[0044] Preparation of copolymer P(MPC-MEONP) containing phosphorylcholine and active ester groups: under nitrogen protection, add 100mL absolute ethanol, 2.58g p-nitrophenoxyformylmethylpropene to a 250mL three-necked bottle Acyl polyethylene glycol ester (MEONP, polyethylene glycol carbon atom is 6), 2.95g methacryloyloxyethyl phosphorylcholine (MPC) and azobisisobutyronitrile 0.0522g as polymerization initiator, Reaction at 65°C for 24h. After removing two-thirds of the solvent by evaporation under reduced pressure, precipitation was carried out with diethyl ether 4 times the volume of the solution to obtain a pale yellow solid, namely P(MPC-MEONP). 1 The molar contents of MPC and MEONP components were determined to be 69% and 31% by H NMR.
Embodiment 2
[0046] Preparation of PMPA containing phosphorylcholine, active ester group and hydrophobic group copolymer: under nitrogen protection, add 150mL absolute ethanol, 1.76g p-nitrophenoxyformyl methacrylic acid to a 250mL three-necked bottle Acyl polyethylene glycol ester (MEONP, polyethylene glycol carbon atoms are about 200), 3.98g methacryloyloxyethyl phosphorylcholine (MPC), 1.27g lauryl methacrylate (LMA) With 0.0703 g of azobisisobutyronitrile as a polymerization initiator, react at 70° C. for 24 hours. After removing two-thirds of the solvent by evaporation under reduced pressure, dialyze with a dialysis bag with a molecular weight cut-off of 6000 for 2 days, and freeze-dry the solution in the dialysis bag to obtain light yellow PMPA solid. 1 The molar percentages of MPC, MEONP and LMA in the polymer PMPA were measured by H NMR to be 61%, 18% and 21%, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com