Novel process for preparing battery-grade iron phosphate material by using iron hydroxide
A technology of iron oxyhydroxide and iron phosphate, applied in phosphorus compounds, inorganic chemistry, chemical instruments and methods, etc., can solve the problems of difficult composition control, large discharge of three wastes, and high production cost, and achieves simple and easy process, less impurities, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
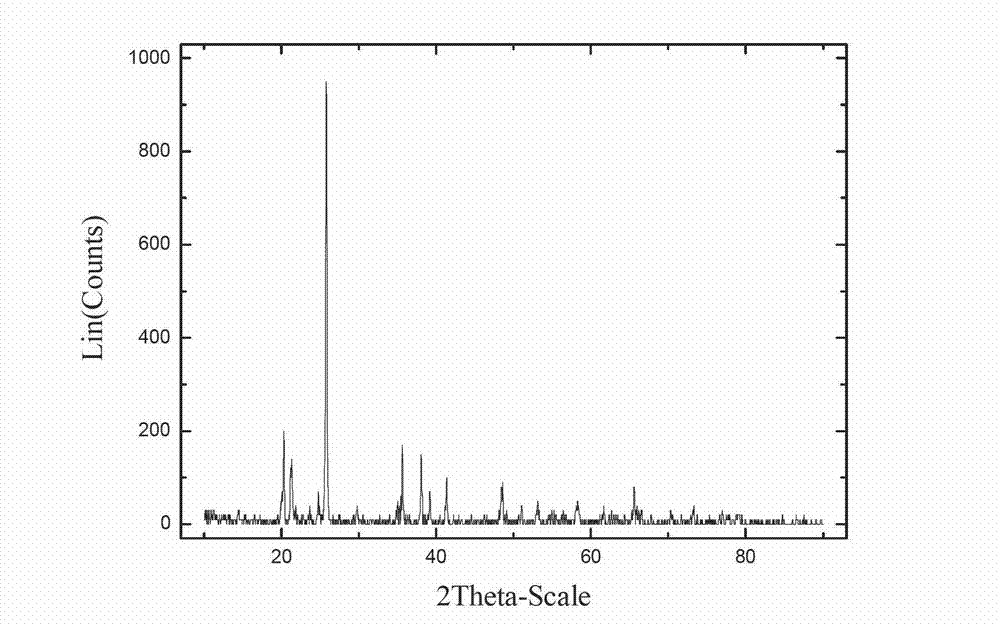
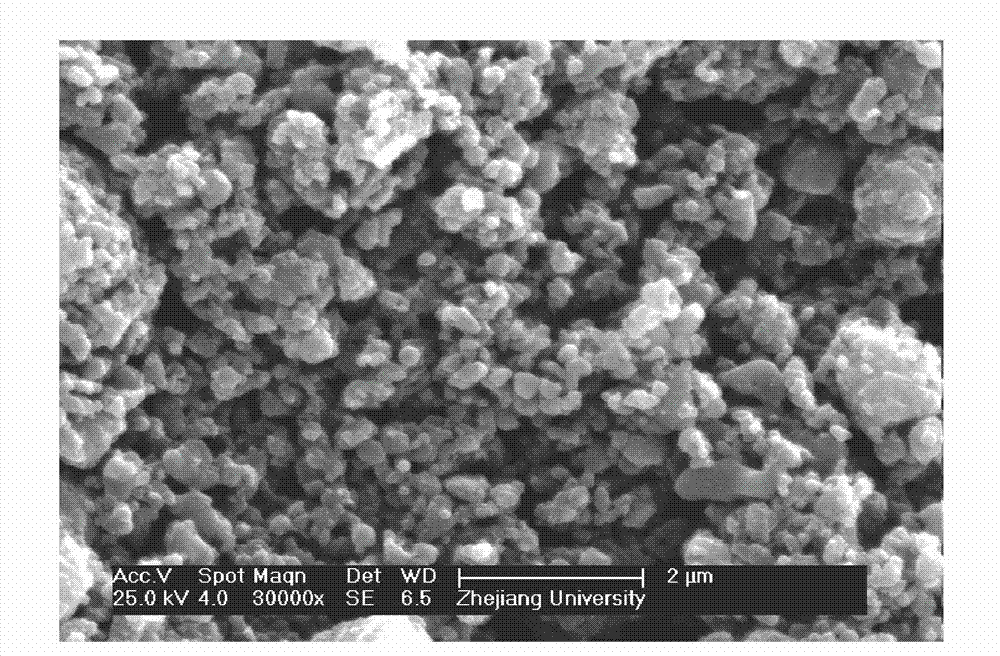
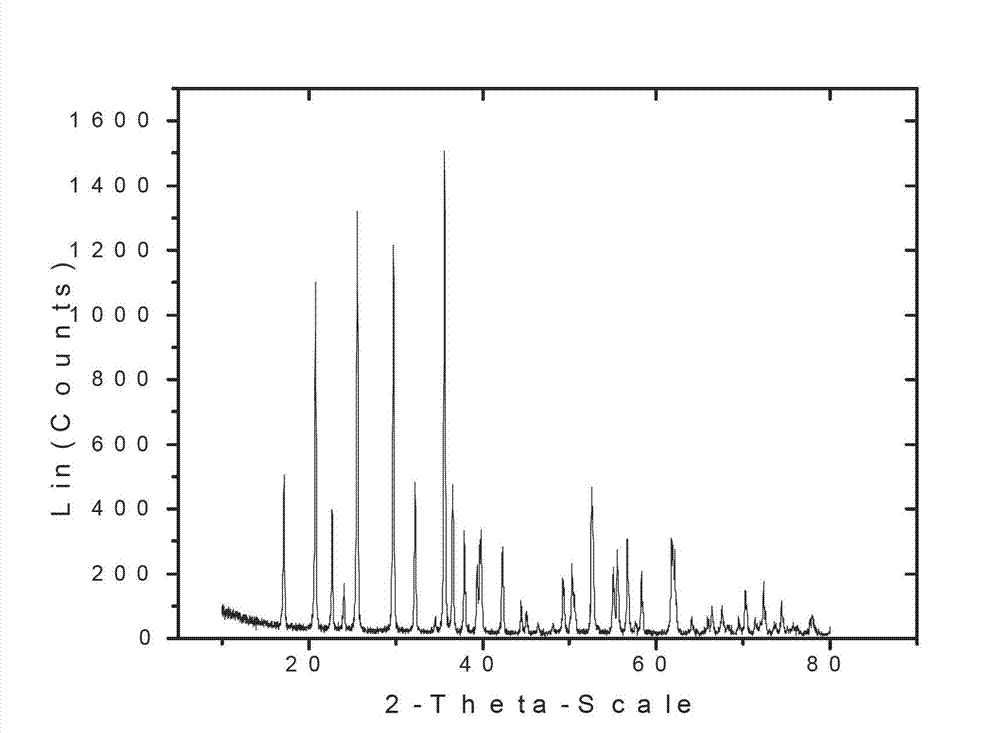
[0025] Embodiment 1, with reference to Figure 1-4 :
[0026] Put 2.8g of reduced iron powder (98% iron content), 6g of glacial acetic acid and 10g of citric acid in 200 ml of primary water for 2 hours. After the reaction of the reduced iron powder is complete, slowly add hydrogen peroxide (mass fraction 10%) for 2 hours until the source of zero-valent iron disappears and the solution turns orange, at which point iron oxyhydroxide is formed. Then 0.14 g of sodium dodecylbenzenesulfonate was added to the solution. After diluting 5.65g of concentrated phosphoric acid to 2 times the volume, slowly add it into the solution under stirring conditions, add dilute ammonia water to adjust the pH value to 1.5, and obtain iron phosphate precipitation. Finally, wash 4 times with 52g of water once and dry in vacuum at 90°C for 4 hours to obtain the product FePO 4 2H 2 O. X-ray powder diffraction analysis was carried out after sintering at 600°C for 6 hours, and the results showed that...
Embodiment 2
[0028] Put 14g of reduced iron powder (98% iron content), 15g of dilute hydrochloric acid, 0.56g of ethylene acid and 26.3g of citric acid in 2000ml of primary water for 2 hours. Then slowly add hydrogen peroxide (mass fraction 10%), and add hydrogen peroxide for 3 hours until the reaction of the reduced iron powder is complete, at which time iron oxyhydroxide is formed. . Another 0.14 g of Tween-80 was added to the solution. After diluting 28.25g of concentrated phosphoric acid to 3 times the volume, slowly add it into the solution under stirring conditions, add dilute ammonia water to adjust the pH value to 1.8, and obtain iron phosphate precipitation. Finally, wash 3 times with 112g of water once and dry in vacuum at 50°C for 12 hours to obtain the product FePO 4 2H 2 O.
Embodiment 3
[0030] 14g of reduced iron powder (98% iron content), 45g of dilute sulfuric acid and 5g of citric acid were stirred and reacted in primary water 2000ml for 4 hours. Then slowly add hydrogen peroxide (mass fraction 10%), and add hydrogen peroxide for 3 hours until the reaction of the reduced iron powder is complete, at which time iron oxyhydroxide is formed. Another 0.3 g of sodium lauryl sulfate was added to the solution. After preparing 32 g of ammonium phosphate into a 1 mol / L solution, slowly add it into the solution under stirring conditions to obtain iron phosphate precipitation, and add dilute ammonia water to adjust the pH value to 1.6. Finally, wash 4 times with 150g of water once and dry in vacuum at 80°C for 10 hours to obtain the product FePO 4 2H 2 O.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com