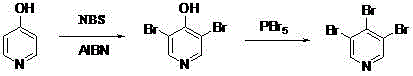
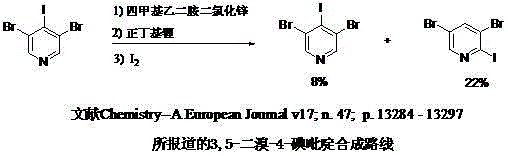
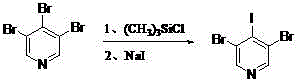Synthetic method for 3, 5-dibromo-4-iodopyridine catalyzed by alkyl silicon reagent
A technology of iodine pyridine and silicon reagent, applied in the synthesis field of halopyridine, can solve the problems of large steric hindrance at the 4-position of tribromopyridine, low reactivity of bromopyridine, difficult separation and purification, etc. Economic and social benefits, easy purification, high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
[0025] Synthesis of 3,5-dibromo-4-hydroxypyridine: add solvent carbon tetrachloride 900ml, 4-hydroxypyridine 95.10g (1.0mol), azobisisobutyronitrile (AIBN) to a 2000ml three-necked flask in sequence 0.82g, add 391.56g (2.2mol) of N-bromosuccinimide (NBS) in batches at 20°C, and react at room temperature for 24 hours. The raw material 4-hydroxypyridine and the intermediate 3- After the bromo-4-hydroxypyridine was converted into the target product 3,5-dibromo-4-hydroxypyridine, the reaction was stopped. Post-reaction treatment: After stirring the reaction liquid to cool to room temperature, pour it into 1000ml carbon tetrachloride, stir, filter, wash the filter cake 3 times with 3*300ml carbon tetrachloride, and wash the filtrate once with aqueous sodium bicarbonate solution. Saturated brine was washed once, and the solvent carbon tetrachloride was removed by rotary evaporation to obtain a crude product of 3,5-dibromo-4-hydroxypyridine, which was recrystallized from ...
Embodiment 2
[0028] In a 2000ml three-necked reaction flask, under the protection of nitrogen, add 800ml of anhydrous propionitrile, 124.5g of potassium iodide (0.75mol, 1.5eq), and 59.8g of trimethylchlorosilane (0.55mol, 1.1eq) in sequence And 157.9g (0.50mol, 1.3eq) of the 3,4,5-tribromopyridine intermediate prepared according to the method in Example 1, heated to reflux for 6 hours, and monitored by high performance liquid chromatography. Cool the bath to 0°C, pour the reaction solution into 1000ml of ice, adjust the pH to 7.0 with solid sodium hydroxide, add the extractant 3*600ml of ethyl acetate, extract 3 times, separate the phases, and wash the organic phase with saturated brine Once, separate the phases, dry over anhydrous sodium sulfate, filter, remove the solvent under reduced pressure, and recrystallize with petroleum ether to obtain 156.2 g of pure white 3,5-dibromo-4-iodopyridine, with a yield of 86.1%. 98.7%.
Embodiment 3
[0030] In the reaction, 37.9 g (0.35 mol, 0.7 eq) of trimethylchlorosilane was used, and the other operations were the same as in Example 2.
[0031] According to the operation in Example 2, 131.0 g of pure white 3,5-dibromo-4-iodopyridine was obtained, with a yield of 72.2% and a content of 97.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com