Poly (gamma-propargyl-L-glutamate)-polyamino acid segmented copolymer, functional segmented copolymer and preparation method
A technology of block copolymer and glutamic acid ester, applied in the field of polymers, can solve the problems of polyamino acid main chain breakage and low reactivity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
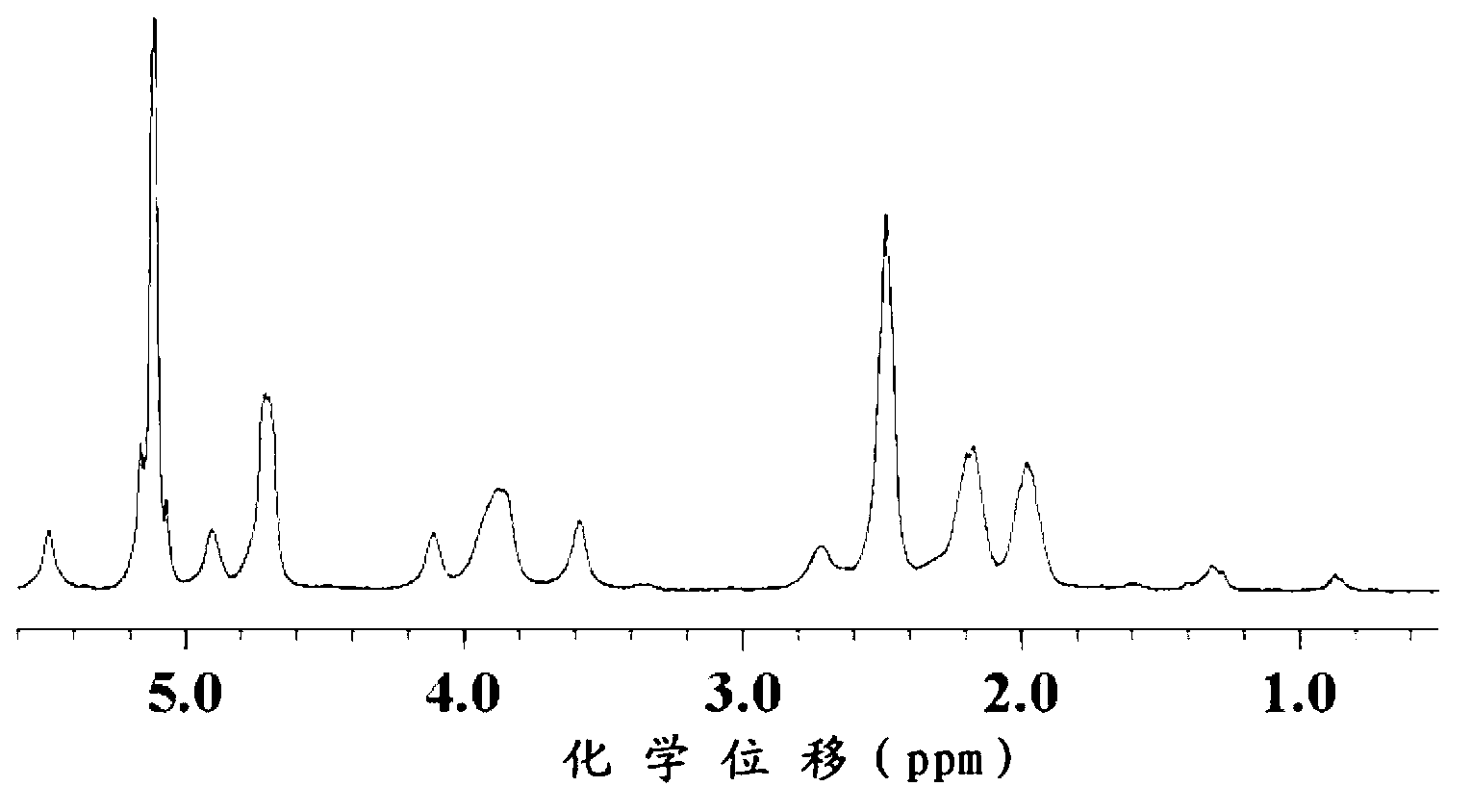
Image
Examples
preparation example Construction
[0063] The invention provides a kind of preparation method of poly(γ-propargyl-L-glutamate)-polyamino acid block copolymer, comprising the following steps:
[0064] γ-propargyl-L-glutamic acid ester-N-internal carboxylic anhydride, amino acid-N-internal carboxylic anhydride and primary amine initiator are polymerized in anhydrous solvent to obtain poly(γ-propargyl- L-glutamate)-polyamino acid block copolymer;
[0065] The number of primary amines of the primary amine initiator is 1 to 2, and the molecular weight of the primary amine initiator is 50 to 1000;
[0066] The amino acid-N-internal carboxylic anhydride is γ-benzyl-L-glutamate-N-internal carboxylic anhydride, γ-propynyl-L-glutamate-N-internal carboxylic anhydride, γ- 2-Chloroethyl-L-glutamate-N-endocarboxylic anhydride, glycine-N-endocarboxylic anhydride, L-alanine-N-endocarboxylic anhydride, L-valine-N-endocarboxylic anhydride , L-leucine-N-endocarboxylic anhydride, L-isoleucine-N-endocarboxylic anhydride, L-phenyl...
Embodiment 1~5
[0101] Weigh 0.1056g (0.0005mol), 0.1056g (0.0005mol), 0.1056g (0.0005mol), 2.1117g (0.01mol), 4.2234g (0.02mol) of γ-propynyl-L-glutamate -N-internal carboxylic acid anhydride was placed in 5 anhydrous reaction flasks, and 5 mL of anhydrous N,N-dimethylformamide was added to dissolve the monomers. Then 10.119mg (0.0001mol) of n-hexylamine were added thereto under stirring, and the resulting mixed solution was stirred and reacted at 25°C for 72h, and then 0.1316g (0.0005mol), 2.6325g (0.01 mol), 5.265g (0.02mol), 2.6325g (0.01mol), 2.6325g (0.01mol) of γ-benzyl-L-glutamate-N-internal carboxylic acid anhydride, continue to react for 72h, after the end of the reaction , the reaction system was settled with 50mL ether, filtered, washed three times with ether, and dried in vacuum at 25°C for 24h to obtain the reaction product.
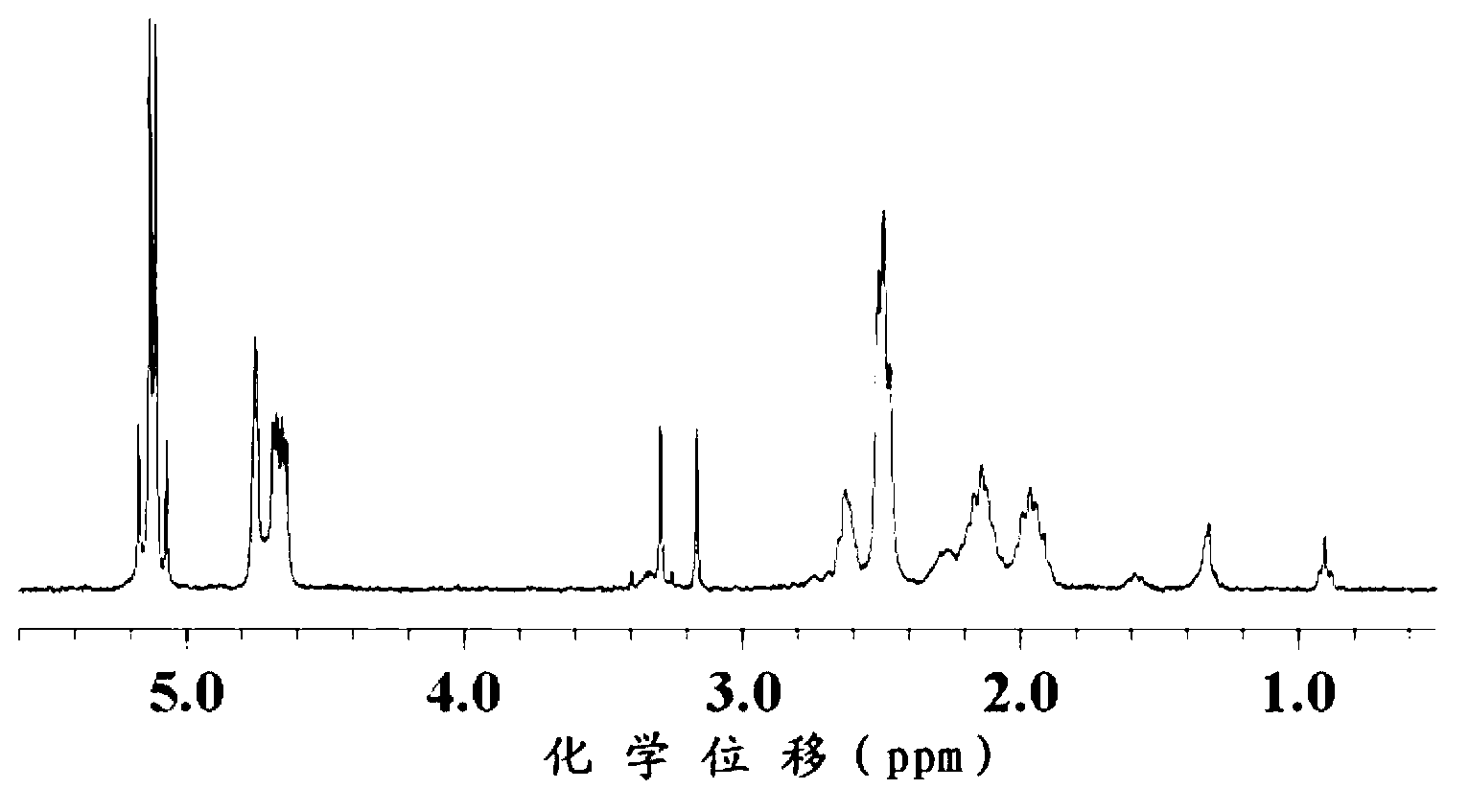
[0102] The present invention carries out structural identification to the product that obtains, and the result is as follows figure 1 as shown, figure ...
Embodiment 6~10
[0110] Take respectively poly(γ-propargyl-L-glutamate)-poly(γ-benzyl-L-glutamate) block copolymer 0.1g that embodiment 1~5 obtains, its Dissolve in 1mL of dichloroacetic acid at 25°C, and then add 0.3mL of a mixed solution of hydrogen bromide and glacial acetic acid with a mass percentage of hydrogen bromide of 33% under stirring to obtain a reaction mixture, and mix the obtained reaction The solution was stirred at 25° C. for 1 h to obtain a reaction solution. The resulting reaction solution was settled with 15 mL of ether, filtered, washed three times with ether, and dried under vacuum at 25° C. for 24 h to obtain a reaction product.
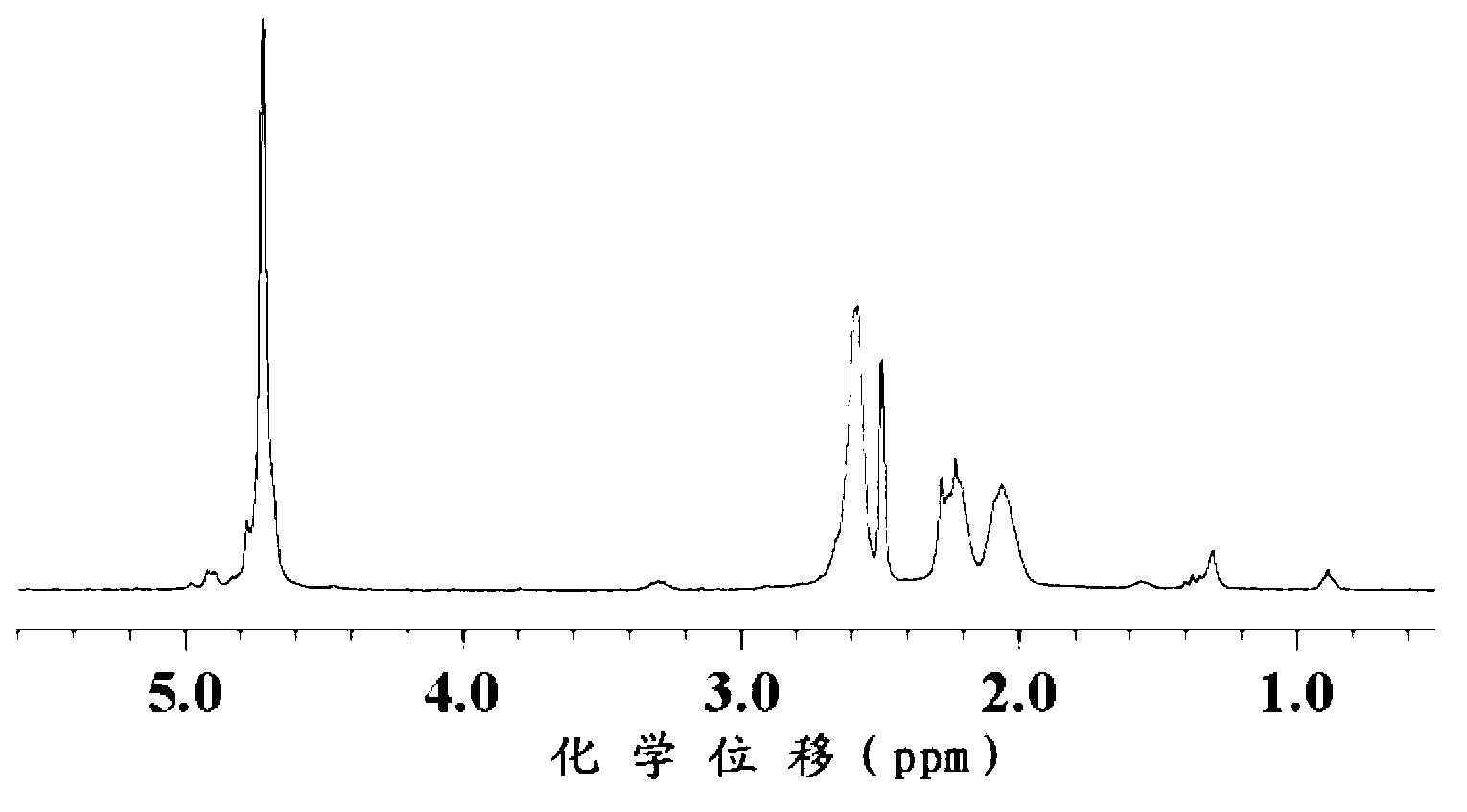
[0111] In the present invention, the obtained reaction product is detected by proton nuclear magnetic resonance spectrum, and the results are as follows: figure 2 as shown, figure 2 It is the H NMR spectrum of the block copolymer obtained in Example 7 of the present invention. Depend on figure 2 It can be seen that 5.17ppm~5.07ppm is th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com