Chargeable alkali metal-sulfur liquid flow battery
A flow battery and alkali metal technology, applied in the field of alkali metal-sulfur flow batteries, can solve the problems of rising internal resistance, falling specific energy, low charging and discharging efficiency, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
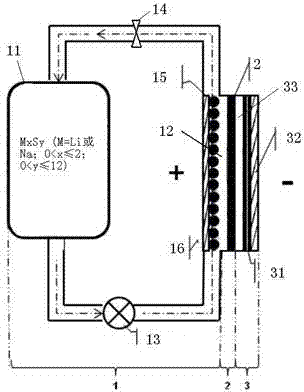
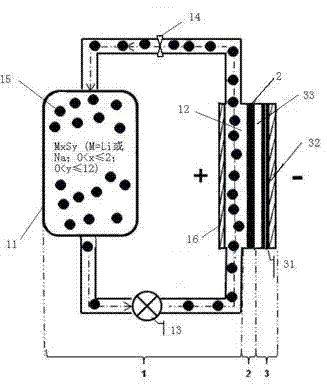
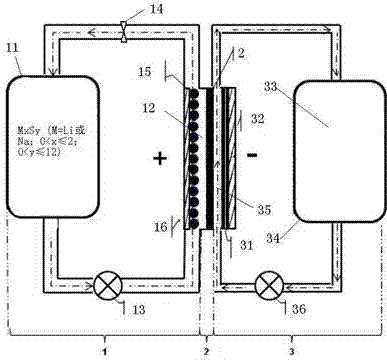
[0044] Such as figure 1 As shown, the rechargeable alkali metal-sulfur flow battery in this embodiment includes: a positive electrode chamber part 1 , a diaphragm 2 , and a negative electrode chamber part 3 . Wherein, the positive electrode partial chamber 1 mainly includes a liquid storage tank 11 , a positive electrode reaction chamber 12 , and a piston pump 13 and a flow valve 14 arranged on the pipeline connecting the liquid storage tank 11 and the reaction chamber 12 . The positive electrode is a positive electrode slurry circulating between the positive electrode reaction chamber 12 and the liquid storage tank 11, and the positive electrode slurry is composed of a positive electrode electrolyte solution and a positive electrode active material mixed in the positive electrode electrolyte solution, wherein the positive electrode active material is M x S y One or more of (M=Li or Na; 0<x≤2; 0<y≤12), during work, it needs to be pumped into the positive electrode reaction ch...
Embodiment 2
[0055] The structure of the battery in this example is basically the same as that of Example 1, the difference is that the composition of the positive electrode slurry used in this example is different from that of Example 1, specifically:
[0056] Positive reaction chamber 1:
[0057] The positive electrode is a positive electrode slurry that circulates between the positive electrode chamber and the liquid storage tank. The positive electrode electrolyte: its solvent is an organic solvent DOL:DME=1:1, and the electrolyte is lithium salt LiBF 4 . The concentration is 1mol / L.
[0058] In the positive electrode electrolyte, the positive electrode active material is Li with a molar ratio of 1:11. 2 S: S, mixed to form a positive electrode slurry, the active material concentration is 1mol / L Li 2 S 12 .
[0059] In the positive electrode reaction chamber, the current collector is also provided with acetylene black, a positive electrode conductive material. The specific setting...
Embodiment 3
[0065] The structure of the battery in this example is basically the same as that of Example 1, the difference is that the composition of the positive electrode slurry used in this example is different from that of Example 1, specifically:
[0066] Positive reaction chamber 1:
[0067] The positive electrode is a positive electrode slurry that circulates between the positive electrode chamber and the liquid storage tank. The positive electrode electrolyte: its solvent is an organic solvent DOL:DME=1:1, and the electrolyte is lithium salt LiBF 4 . The concentration is 1mol / L. In the positive electrode electrolyte, add the positive electrode active material molar ratio of Li 2 S: S, mixed to form a positive electrode slurry, the active material is added to the positive electrode slurry with a concentration of 0.5mol / L Li 2 S 10 .
[0068] In the positive electrode reaction chamber, acetylene black, a positive electrode conductive material, is also arranged on the positive e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com