Preparation method for compound cortisone acetate
A technology of cortisone acetate and compound, applied in the field of preparation of pine acetate, can solve the problems of long synthesis steps, low yield, and high production requirements, and achieves reducing process cost, improving product yield, improving quality and Yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
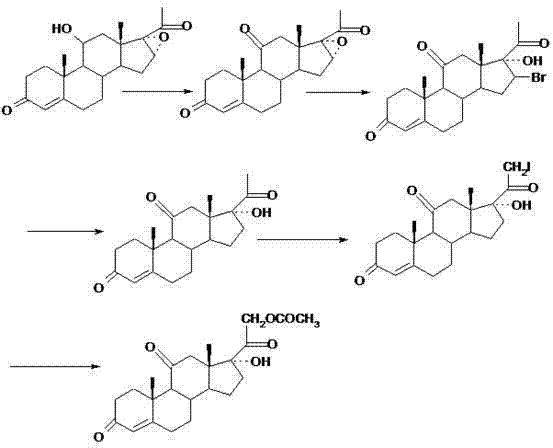
[0015] The preparation method of the compound cortisone acetate of the present invention comprises the following steps:
[0016] The first step is to carry out hydroxyl oxidation on the 11th position;
[0017] Put the compound 4-pregnene-11α, 17α-alcohol-3,20-dione (I) and the oxidation reaction solvent into it, stir to cool down, add the oxidation reagent dropwise, and the dropwise addition is completed in about 1 to 3 hours; The reaction is incubated at the temperature for 1.5 to 2 hours. After the insulation is completed, an oxidizing reagent quencher is added, and the material is pulled into a concentrated water separation tank. After concentration, water is added and stirred for water separation. The material is filtered and dried to obtain the compound 4-pregnene-17α-ol -3,11,20-triketone (II);
[0018] Oxidation reaction solvent can be a kind of in acetone, glacial acetic acid, methylene dichloride, preferably acetone;
[0019] Oxidizing reagent quencher can be a kind...
Embodiment 1
[0030] Preparation process of compound 4-pregnene-21-iodo-17α, 21-diol-3,11,20-triketo-21-acetate (IV):
[0031] Put 11g of chromic anhydride and 33ml of water into the oxidation reagent preparation bottle, stir to dissolve, cool down to below 10°C, add 8.8ml of concentrated sulfuric acid dropwise, set aside; put acetone and 4-pregnene-11α into the oxidation reaction bottle, 17α-alcohol-3,20-dione (I) 50g, stir to cool down; when the temperature drops to 10~20℃, add the oxidizing reagent dropwise, control the dropwise temperature at 5~10℃, and complete the dropwise addition in about 2 hours; After the reaction is kept at a temperature of 5 to 10 ° C for 2 hours, 10 g of sodium sulfite and 100 ml of water are added after the insulation is completed, and stirring is continued for 15 minutes. Oxide 4-pregnene-17α-ol-3,11,20-trione (II) 47.5g, yield 95%, purity 98.8%;
[0032] Put 30ml of methanol and 3g of anhydrous calcium chloride into the reaction flask, stir and dissolve, co...
Embodiment 2
[0034] Preparation process of compound 4-pregnene-21-iodo-17α, 21-diol-3,11,20-triketo-21-acetate (IV):
[0035] Put 12g of chromic anhydride and 36ml of water into the oxidation reagent preparation bottle, stir and dissolve, cool down to below 10°C, add 9.6ml of concentrated sulfuric acid dropwise, set aside; put 200ml of acetone, 4-pregnene-11α into the oxidation reaction bottle , 50 g of 17α-alcohol-3,20-dione (I), stir to cool down; when the temperature drops to 10~20 ℃, add the oxidizing reagent dropwise, the dropwise temperature is controlled at 10~20 ℃, and the dropwise addition is completed in about 1 hour; After dripping, the reaction was incubated for 2 hours at a temperature of 10 to 20°C. After the insulation was completed, 20 ml of isopropanol was added, and stirring was continued for 15 minutes. After that, the mixture was concentrated under reduced pressure to a solvent-free state, and then lowered to normal temperature. Obtained oxide 4-pregnene-17α-ol-3,11,20-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com