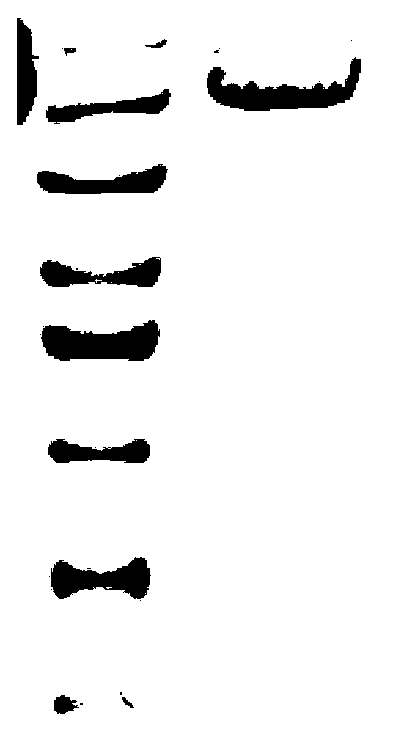Recombinant protein HF2 used for methicillin-resistant staphylococcus aureus (MRSA) vaccine, and preparation method and application thereof
A technology of recombinant protein and staphylococcus, applied in the biological field, can solve the problems of few treatment methods and difficult control of the situation, and achieve the effect of easy separation and purification, simple steps, and good immune protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1: The DNA sequence of methicillin-resistant Staphylococcus aureus Hla (H35L delta110-150) was synthesized by Shanghai Jierui Biotechnology Co., Ltd.
Embodiment 2
[0055] Example 2: PCR method was used to amplify the H portion used to construct HF2.
[0056] 1. Considering the connecting peptide SEQ ID NO:3 of the H segment, design the following primers to clone the H gene from the synthetic DNA sequence:
[0057] SEQ ID NO: 7gc ggatcc at ggcagattct gatattaataataa (underlined restriction restriction site BamHI)
[0058] SEQ ID NO:8aactaagctgccaccgccac catttgtcat ttc
[0059] 2. PCR clone gene
[0060] PCR system:
[0061] Template (100ng / μl) 0.5μl SEQ ID NO: 7 (50 μM) 1μl SEQ ID NO:8 (50μM) 1μl Primerstar Taq Enzyme 0.5μl dNTP 4μl 5X Buffer 10μl Sterilized double distilled water 33μl total capacity 50μl
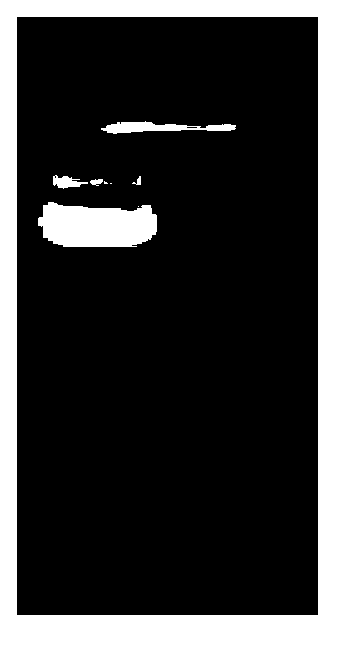
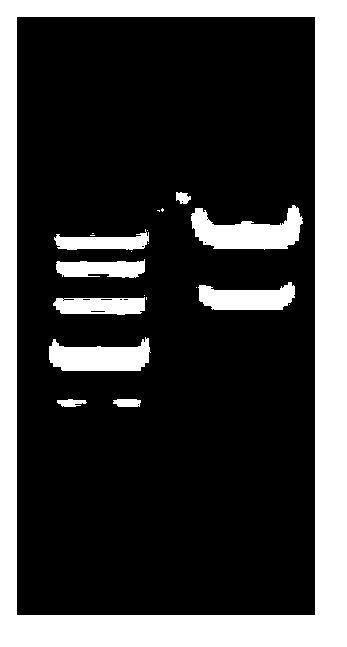
[0062] The PCR amplification reaction conditions were denaturation at 98°C for 10s, annealing at 60°C for 10s, extension at 72°C for 1min and 30s, 30 cycles, and complete extension at 72°C for 6min. After the reaction was completed, the PCR amplification results wer...
Embodiment 3
[0064] Example 3: Cloning of Methicillin-resistant Staphylococcus aureus Fibronectin Binding Protein A Active Fragment FNBA2 Active Fragment (F2)
[0065] 1. First, according to the full-length gene sequence of MRSA-252FnBA protein, use bioinformatics software for structural analysis to determine the FnBA1 target gene fragment that needs to be amplified.
[0066] 2. According to the analysis results, the PCR method was used to amplify the FnBA1 target gene fragment from the MRSA-252 genome, and the amplification steps were as follows:
[0067] 1) Design PCR primers as follows, respectively SEQ ID NO: 9-10
[0068] SEQ ID NO: 9ggtggcggtg gcagcttagt ttatatagt aataaagc
[0069] SEQ ID NO:10ttttcctttt gcggccgc ct aacctttgtt tgttgattct tctc (the restriction site NotI is underlined)
[0070] 2) The methicillin-resistant Staphylococcus aureus MRSA-252 strain was taken out of the -80°C freezer and spread on the MRSA-252 special solid medium, cultivated overnight at 37°C, and then ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com