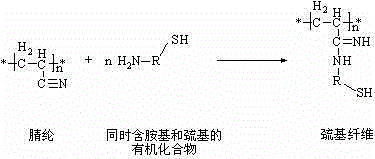
Thiol acrylic fiber material and synthesis method thereof
A technology of acrylic fiber and fiber material, applied in the direction of fiber type, fiber processing, textile and paper making, etc., to achieve the effect of good mechanical strength, stable quality and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Put 1.5 g of cysteine hydrochloride, 0.684 g of sodium carbonate, and 150 mL of glycerol into a three-neck flask, stir, and dissolve with slight heat. After the reaction, the fibers were taken out and washed with deionized water until neutral; soaked in 0.2 mol / L hydrochloric acid for 3 h. After the reaction, the fibers were taken out, washed with deionized water until neutral, and dried at 45°C to constant weight. The weighed fiber mass was 1.139 g, which was 13.9% heavier than before the reaction, and the grafted amount was 0.139 g·g -1 fiber. The mercapto group content of the fibers synthesized in this example was measured to be 0.46 mmol g by indirect iodometric method (Journal of Hazardous Materials, 2009, 168, 1575-1580). -1 .
Embodiment 2
[0019] Put 10 g of cysteine hydrochloride, 4.6 g of sodium hydroxide, and 250 mL of glycerol into a three-neck flask and stir, and dissolve it by slight heating. Put in 10 g of acrylic fiber, and react at a temperature of 130°C for 4.5 h. After the reaction, the fibers were taken out and washed with deionized water until neutral; soaked in 0.2 mol / L hydrochloric acid for 3 h, after the reaction was completed, the fibers were taken out, washed with deionized water until neutral, and dried at 45°C to constant weight . The weighed fiber mass was 13.430 g, which was 34.3% heavier than before the reaction, and the grafted amount was 0.343 g·g -1 fiber. The mercapto group content of the fibers synthesized in this example was measured to be 1.56 mmol g by indirect iodometric method (Journal of Hazardous Materials, 2009, 168, 1575-1580). -1 .
Embodiment 3
[0021] Put 5 g of mercaptoethylamine hydrochloride, 1.76 g of potassium hydroxide, and 100 mL of ethylene glycol into a three-necked flask, stir, heat slightly to dissolve, add 5 g of acrylic fibers, and react at a temperature of 125-130°C for 3 h After the reaction, the fibers were taken out and washed with deionized water until neutral; soaked in 0.5 mol / L hydrochloric acid for 3 h, after the reaction, the fibers were taken out, washed with deionized water until neutral, and dried at 45°C until constant Heavy. The weighed fiber mass was 7.965 g, which was 59.3% heavier than before the reaction, and the grafted amount was 0.593 g·g -1 fiber. The mercapto group content of the fibers synthesized in this example was measured to be 5.08 mmol g by indirect iodometric method (Journal of Hazardous Materials, 2009, 168, 1575-1580). -1 .
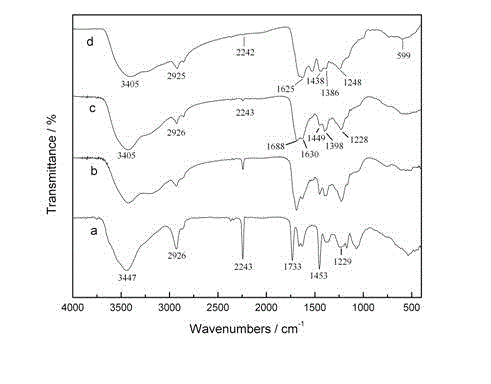
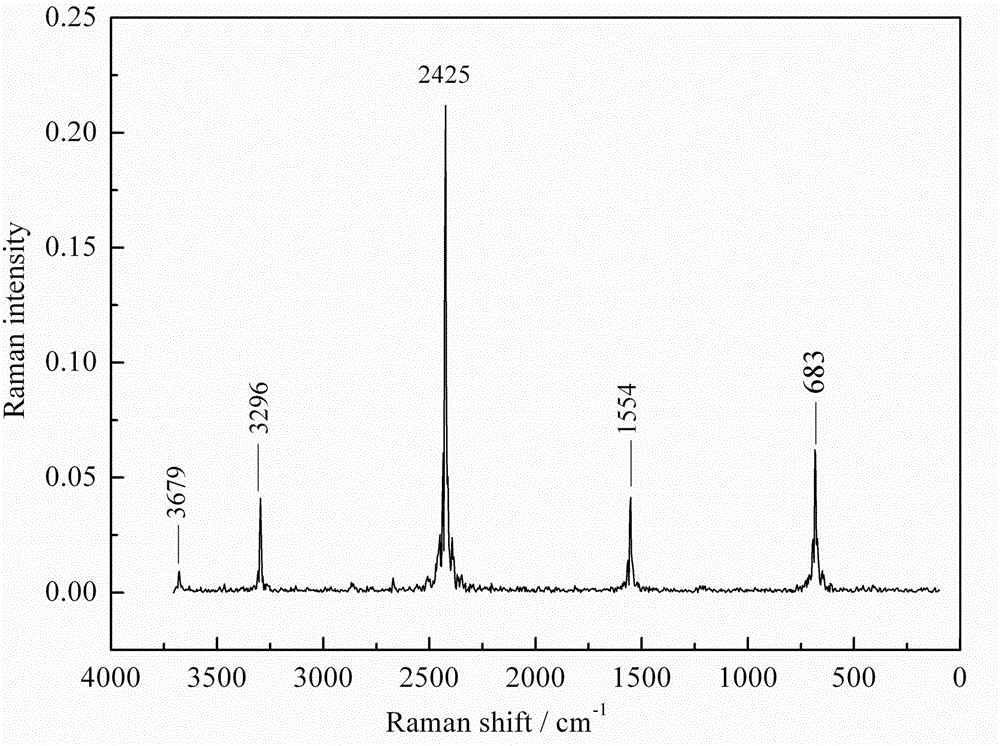
[0022] The infrared spectrum of the synthetic fiber in embodiment 1, embodiment 2, embodiment 3 is as attached figure 1 Shown in b, c, d. The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| grafting amount | aaaaa | aaaaa |
| grafting amount | aaaaa | aaaaa |
| grafting amount | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com