Seven-element-ring polyhydroxy nitrone and preparation method and application thereof
A technology of cyclic polyhydroxynitrone and reaction, which is applied in the field of seven-membered cyclic polyhydroxynitrone and its preparation and application, and can solve the problems of no report and inapplicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
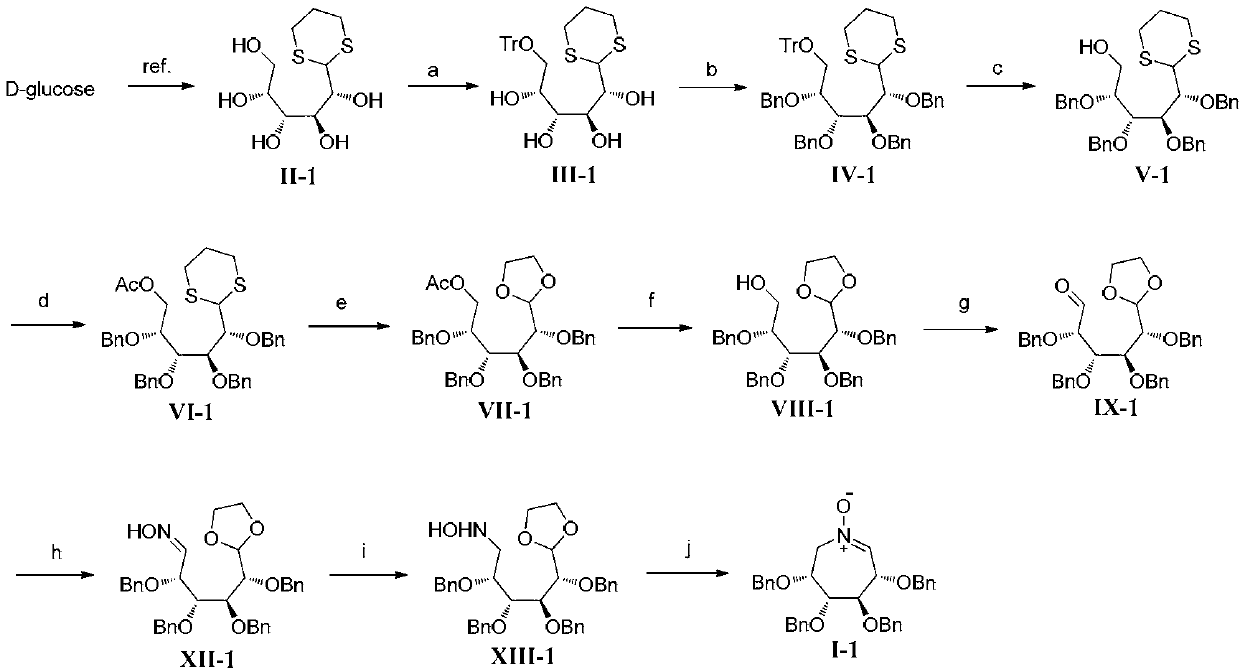
[0088] Embodiment 1, seven-membered ring polyhydroxynitrone I-1 (R in formula I 1 , R 2 , R 3 and R 4 are all benzyl compounds) and the synthesis of its intermediate formula XIII-1 (reaction scheme as figure 1 shown)
[0089]
[0090] According to literature [ O.; Redlich, H.; Frank, H. Synthesis 1995, 1383] Method for the preparation of thioacetal II-1 from D-glucose. D-glucose (10.0 g, 55.6 mmol) was dissolved in concentrated hydrochloric acid (10 mL), 1,3-propanedithiol (5.6 mL, 55.6 mmol) was added, and the reaction mixture was stirred overnight at room temperature. Ethanol (200 mL) was added to the reaction solution, and a large amount of white solid was precipitated. Filter and rinse the filter cake with ethanol. After drying, 13.4 g of a white solid was obtained, namely thioacetal II-1, with a yield of 89%.
[0091] A solution of thioacetal II-1 (11.3g, 42.0mmol), triphenylchloromethane (12.3g, 44.0mmol) and 4-dimethylaminopyridine (51mg, 0.42mmol) in pyridi...
Embodiment 2
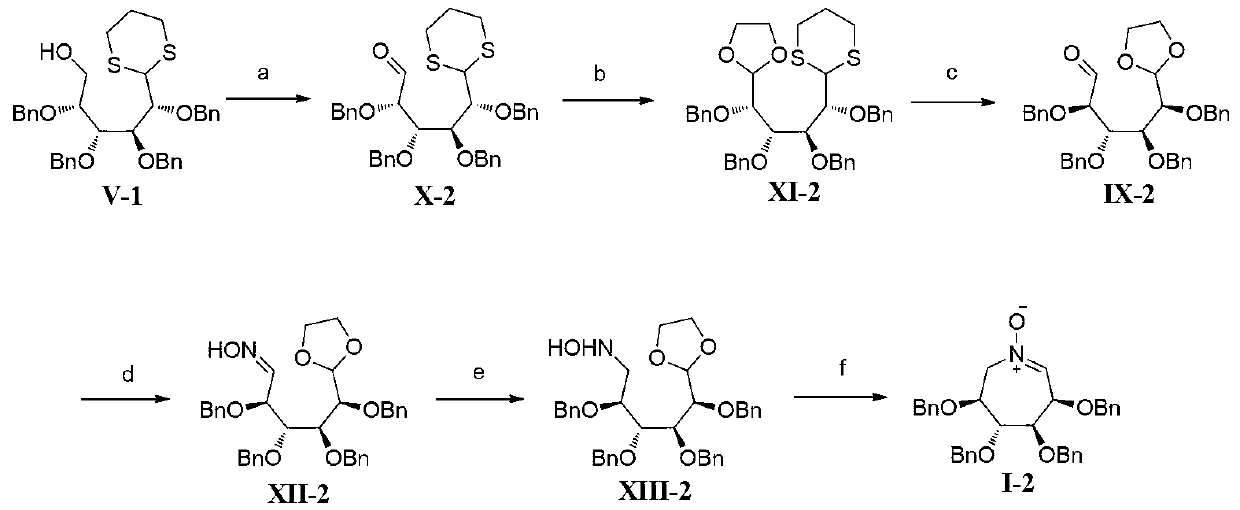
[0103] Embodiment 2, seven-membered ring polyhydroxynitrone I-2 (R in formula I 1 , R 2 , R 3 and R 4 are all benzyl compounds) and the synthesis of intermediates shown in formula XIII-2 (the reaction process is as follows figure 2 shown)
[0104]
[0105] The raw material primary alcohol V-1 was prepared according to the steps in Example 1.
[0106] Dimethylsulfoxide (2.13mL, 30.0mmol) was added dropwise to a solution of oxalyl chloride (1.69mL, 20.0mmol) in dry dichloromethane (50mL) at -70°C. Gas evolved and the mixture was stirred at this temperature for 30 minutes after the addition was complete. A solution of primary alcohol V-1 (6.31 g, 10.0 mmol) in dry dichloromethane (20 mL) was added dropwise to the above reaction system at -70°C, and stirred at this temperature for 30 minutes after the addition was complete. Triethylamine (6.95 mL, 50.0 mmol) was added dropwise at -70°C. After the addition was complete, the mixture was reacted at this temperature for 20 m...
Embodiment 3
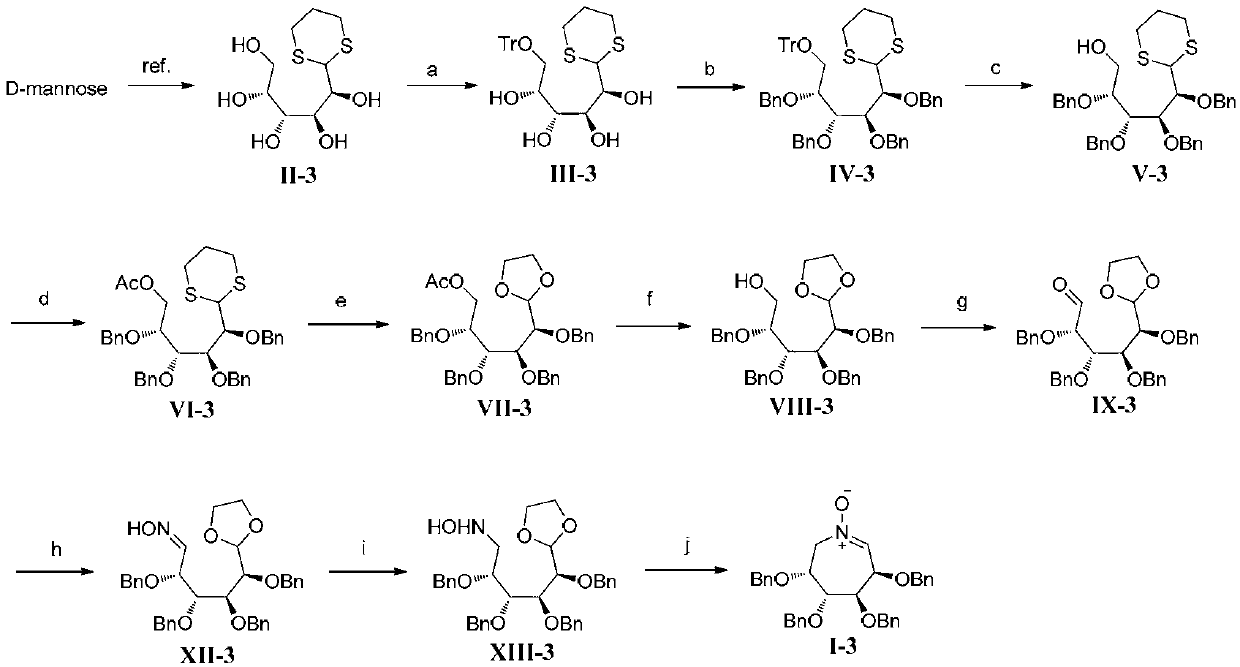
[0114] Embodiment 3, seven-membered ring polyhydroxynitrone I-3 (R in formula I 1 , R 2 , R 3 and R 4 are all benzyl compounds) and the synthesis of intermediates shown in formula XIII-3 (the reaction process is as follows image 3 shown)
[0115]
[0116] According to the literature[ O.; Redlich, H.; Frank, H. Synthesis 1995, 1383] A similar method was used to prepare thioacetal II-3 from D-mannose. D-mannose (9.0 g, 50.0 mmol) was dissolved in 6N hydrochloric acid (20 mL), 1,3-propanedithiol (5.55 mL, 55.0 mmol) was added, and the reaction mixture was stirred overnight at room temperature. The reaction solution was concentrated, recrystallized with ethanol, filtered, and the filter cake was washed with ethanol. After drying, 8.6 g of white solid was obtained, namely thioacetal II-3, with a yield of 65%.
[0117] A solution of thioacetal II-3 (8.1g, 30.0mmol), triphenylchloromethane (8.79g, 31.5mmol) and 4-dimethylaminopyridine (37mg, 0.30mmol) in pyridine (150mL) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com