Preparation method and application of anthraquinone polypyridine ligand and ruthenium-anthraquinone complex
A technology of anthraquinone polypyridine and anthraquinone ruthenium polypyridine is applied in the field of preparation of anthraquinone ligands and anthraquinone ruthenium complexes, can solve problems such as treatment failure, and achieves small molecular structure, good water solubility and simple synthesis method. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
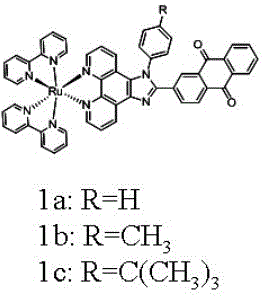
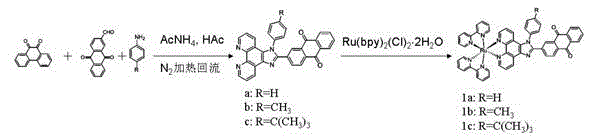
[0034] Example 1 Ligands and Complexes Preparation Methods
[0035] (1) Synthesis of 2-formaldehyde-9,10-anthraquinone
[0036] You can refer to ( J. Org. Chem . 1997, 62 , 5690-5695) synthetic method, in a 150 mL round bottom flask, add 2.0 g (8 mmol) of 2-hydroxymethylanthraquinone, 100 mL of dichloromethane, and then add 2.6 g (12 mmol) of pyridinium chlorochromate Om salt (PCC), stirred at room temperature for 12 h. The excess PCC was filtered off, and the solvent was evaporated under reduced pressure. First wash with 75 mL of distilled water, and then extract with dichloromethane (3×75 mL) three times. The solvent was distilled off under reduced pressure, passed through a silica gel column, and eluted with toluene:dichloromethane=1:5. Yield 98%.
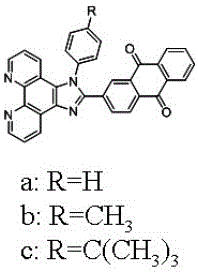
[0037] (2) Synthesis of 2-(phenylimidazo[4,5-f][1,10]phenanthroline-2-)anthraquinone (a)
[0038] Weigh 1,10-phenanthroline dione (0.212 g, 1 mmol), 2-formaldehyde-9,10-anthraquinone (0.236 g, 1 mmol), aniline (0.25 ...
Embodiment 2
[0050] Example 2 Cytotoxicity MTT assay
[0051] Take the tumor cells in the logarithmic growth phase and adjust the cell density to 5×10 3 cells / ml, inoculated in 96-well culture plates, and each sample in the experiment had 6 concentrations of 100, 50, 25, 12.5, 6.25, and 3.125 μM. Four replicate wells were set for each concentration, and more than eight replicate wells were set for the control. Experimental samples were dissolved with DMSO and diluted with DMEM culture medium. After 24 hours of loading, cells were still placed at 37°C, 5% CO 2 , 1%O 2 Continue culturing in an anaerobic incubator for 48 hours, then add MTT, continue culturing for another 4 hours, absorb the supernatant, add 150 μL DMSO to each well, measure the absorbance of each well at a wavelength of 490 nm with an enzyme-linked immunosorbent assay instrument, and calculate the inhibition of cell proliferation Rate. find IC 50 value (drug concentration at which the inhibition rate is equal to 50...
Embodiment 3
[0053] Example 3 Fluorescence imaging experiments of complexes 1a, 1b, 1c in hypoxic cells
[0054] Cell culture: Hela cells were cultured in DMEM medium containing 10% fetal bovine serum, cells (5×10 8 / L) in 6-well plates, 5% CO 2 and 1%O 2 Under anaerobic conditions, 37 o C culture, adherent growth for 24 hours. Then Hela cells were incubated with complexes 1a, 1b, 1c (7.5μM) for 0, 0.5h, 1h, 3h, respectively. Aspirate the culture medium, then wash 2-3 times with PBS buffer, and image under a fluorescent microscope. The result is as Figure 4 shown. With the increase of the incubation time between the complex and the cells, the amount of the complex entering the cells increases, and thus the fluorescence increases.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com