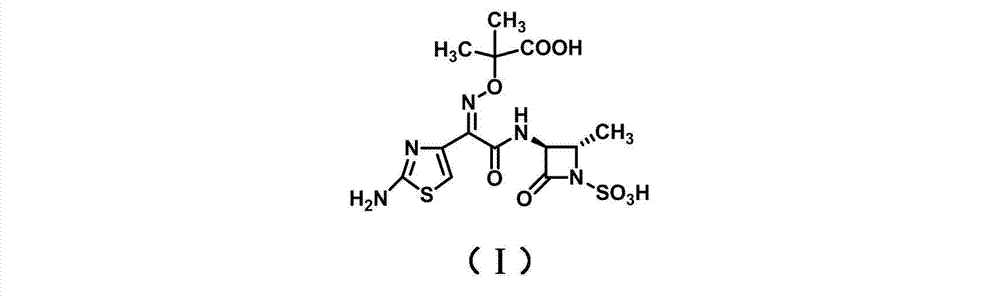
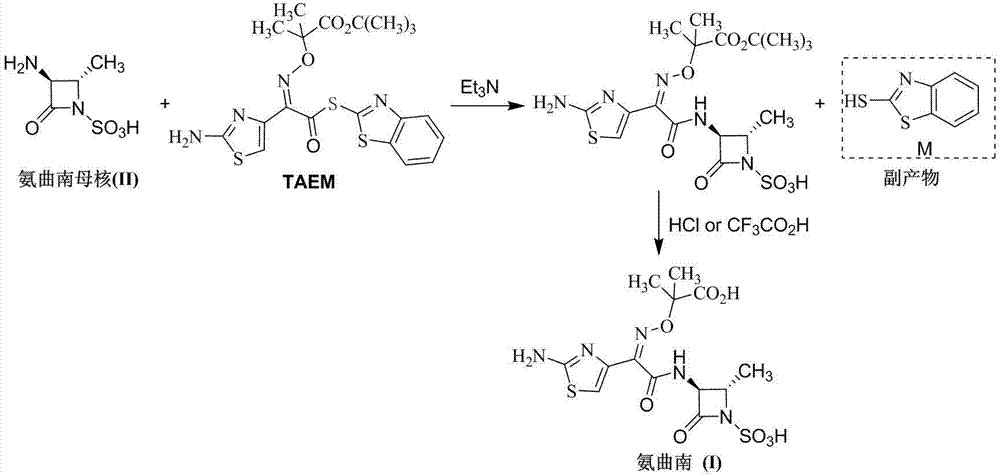
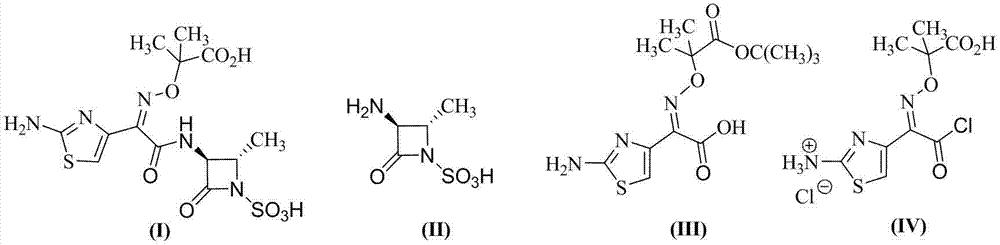
Synthesis method for aztreonam
A synthetic method, the technology of aztreonam, applied in the direction of organic chemistry, etc., can solve the problems of difficult removal of by-product M, large environmental pollution, difficult disposal of waste liquid, etc., to achieve improved yield and purity, good atom economy, The effect of reducing the amount of waste acid
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] In a 500mL three-necked flask, add 50.7g (0.182mol) of triphenylphosphine oxide (TPPO), 100g of dichloromethane, and add 18.1g (0.061mol) of bis(trichloromethyl)carbonate (BTC ) and 50g of dichloromethane, after the dropwise addition, keep warm for 2 hours to prepare a solution containing dichlorotriphenylphosphine.
Embodiment 2
[0030]In a 500mL three-necked flask, add 50.7g (0.182 mol) triphenylphosphine oxide (TPPO), 100g 1,2-dichloroethane, dropwise add 18.1g (0.061 mol) bis(trichloromethyl ) Carbonate (BTC) and 50g of 1,2-dichloroethane solution, after the dropwise addition, keep warm for 2 hours to prepare a solution containing dichlorotriphenylphosphine.
[0031] (2) 2-[[(Z)-1-(2-amino-4-thiazolyl)-2-chloro-2-oxoethylene]amino]oxy-2-methylpropion hydrochloride ( IV) Preparation
Embodiment 3
[0033] In a 1L four-neck flask, add 50.0g (0.152 mol) of compound (III) and 50g of dichloromethane, and add the dichlorotriphenylphosphine solution prepared in Example 1 dropwise at -15°C, and keep the reaction for 3 hours. Raise the temperature to 0°C, inject HCl gas to saturation, and keep stirring for 1 h. Add 400g of petroleum ether and stir for 30min. After suction filtration, the filter cake was vacuum-dried to obtain 45.4 g of compound (IV), with a yield of 82.3%. The mother liquor was evaporated to remove the solvent, and the solid was recrystallized from 300 g of toluene to recover 46.4 g of triphenylphosphine oxide, with a recovery rate of 91.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com