Herpes virus EBV (Epstein-Barr Virus) detection kit
A detection kit and kit technology, applied in the direction of microbial determination/inspection, fluorescence/phosphorescence, biochemical equipment and methods, etc., can solve the problem of low detection sensitivity, achieve high detection sensitivity, wide detection range, and fast operation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
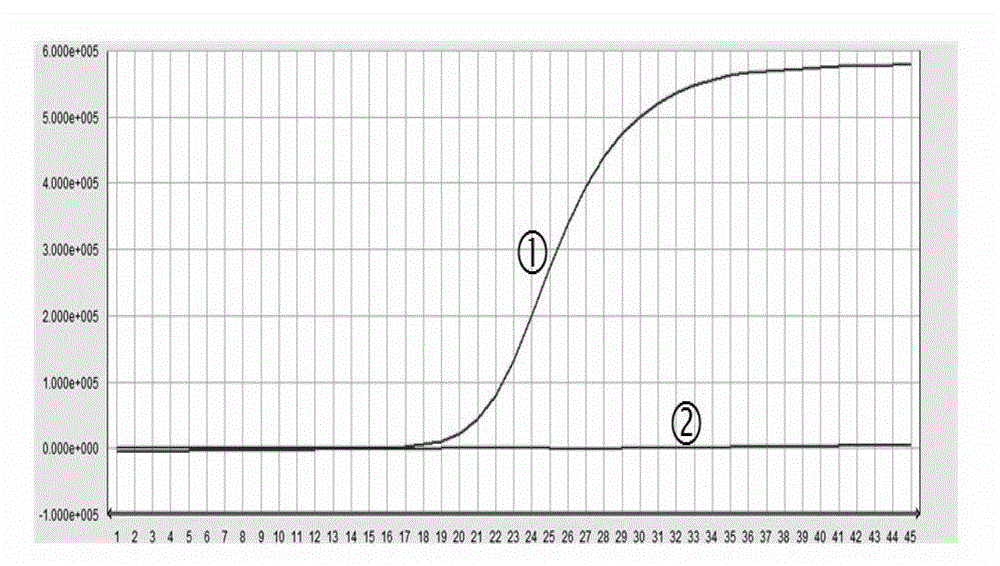
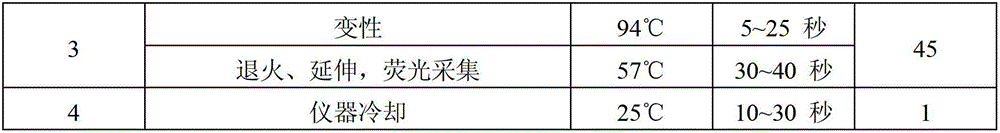
Image
Examples
Embodiment 1
[0024] The present embodiment provides a specific EBV detection kit, which includes the following components:
[0025] ① EBV concentrate: Contains 50mM / L polyethylene glycol 6000 (PEG-6000) and 100mM / L sodium chloride.
[0026] ② Nucleic acid release agent: Contains 0.1mM / L of surfactin, 100mM / L of potassium chloride, 0.1% of sodium dodecylsulfonate (SDS), 0.1% of ethanol and solvent TE buffer.
[0027] ③ Internal standard (positive internal control): It is a recombinant of a 100 base pair artificially synthesized DNA sequence inserted into the pUC18T vector, that is, a plasmid, the concentration is 2.00E+05copies / ml, and the 100 base pair sequence is: 5'-CACCACTTAAATCCTAAGGTTCCAGCTCTGTCATCCAGTTTTGCTGACTCACGTATTC GTAGCCAATCTTCTGGAGGTGCAATCTCAATTATGTCATCAG-3'.
[0028] ④PCR reaction solution: including 5 μl of 10×PCR reaction buffer, 0.2 mmol / L dNTP, 0.3 μmol / L upstream and downstream primers for target polynucleotide amplification, and 0.3 μmol / L probe for target polynucleoti...
Embodiment 2
[0034] This example provides the operation steps for detecting EBV-DNA in unknown samples such as plasma, throat swab, and peripheral blood using the kit described in Example 1 above:
[0035] 1. Reagent preparation
[0036] According to the number of samples to be tested, EBV negative control, EBV positive control, and EBV quantitative reference products A~D, take the corresponding amount of PCR reaction solution (38 μl / person), enzyme mixture (2 μl / person) and content in proportion. Label 1.0 μl / person and mix thoroughly to form a PCR-mix. For example, when the sample to be tested is 3 people, a total of 9 people need to be prepared (the number of people in the above four is 3, 1, 1 and 4 respectively). PCR-mix; ready for use after brief centrifugation.
[0037] 2. Nucleic acid extraction
[0038] 1. Plasma sample: Take 100 μl of plasma sample, add an equal volume of EBV concentrate, centrifuge at 12,000 rpm for 5 minutes, discard the supernatant, add 50 μl of nucleic acid...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com