Method for determining radix notoginseng extract and contents of five types of ginsenosides in preparation of radix notoginseng extract by Fourier transform near-infrared spectrograph
A near-infrared spectrometer and Fourier transform technology, applied in the field of medicine, can solve the problems of large data deviation, long analysis time, large difference in gradient elution parameter parameters, etc. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
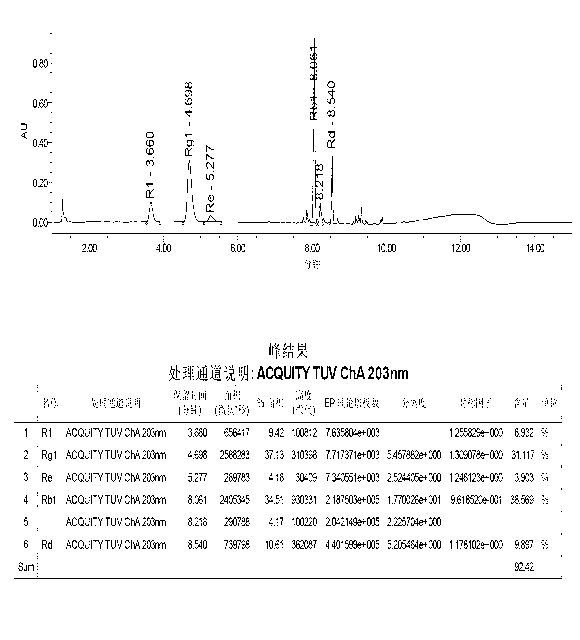
[0052] The method of the present invention is applied to five kinds of saponins (notoginseng saponin R 1 , Ginsenoside Rg 1 , Ginsenoside Re, Ginsenoside Rb 1 , ginsenoside Rd) content determination, the steps are as follows:
[0053] (1) Collect samples of Panax notoginseng total saponins:
[0054] 100 batches of Panax notoginseng saponins were collected, 70 batches were randomly selected as calibration sample sets for modeling, and the remaining 30 batches were used as verification sample sets to verify the established calibration model.
[0055] (2) Collect the near-infrared diffuse reflectance spectrum of Panax notoginseng saponins:
[0056] Collect the near-infrared spectra of each sample in the calibration sample set and the near-infrared spectrum of each sample in the verification sample set described in step (1), which are the original spectra of the calibration sample set and the original spectra of the verification sample set;
[0057] Scanning conditions: use a di...
Embodiment 2
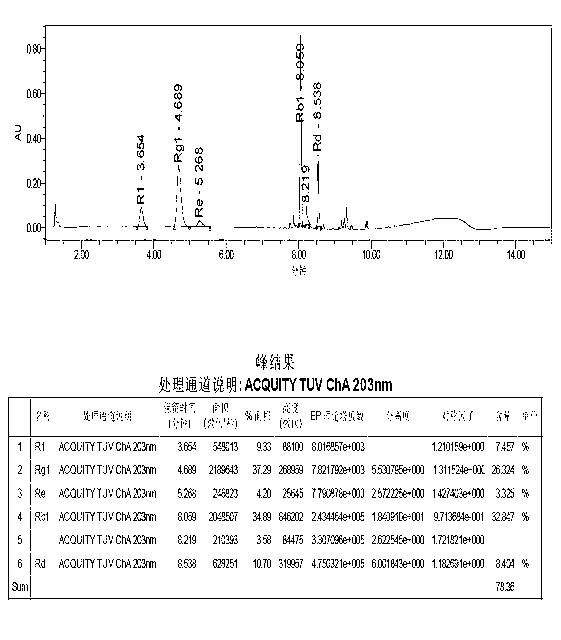
[0136] The method of the present invention is applied to five kinds of saponins (notoginseng saponin R 1 , Ginsenoside Rg 1 , Ginsenoside Re, Ginsenoside Rb 1 , ginsenoside Rd) content determination, the steps are as follows:
[0137] (1) A total of 100 batches of injection samples prepared from Panax notoginseng extract were collected, 68 batches were randomly selected as calibration sample sets for modeling, and the remaining 32 batches of injection samples prepared from Panax notoginseng extract were used as verification sample sets, Used to validate the established calibration model.
[0138] (2) Collect the near-infrared transmission spectrum of the injection prepared from the Panax notoginseng extract;
[0139] Collecting the near-infrared transmission spectrum of each sample in the calibration sample set and the near-infrared transmission spectrum of each sample in the verification sample set described in step (1) respectively are the original spectrum of the calibra...
Embodiment 3
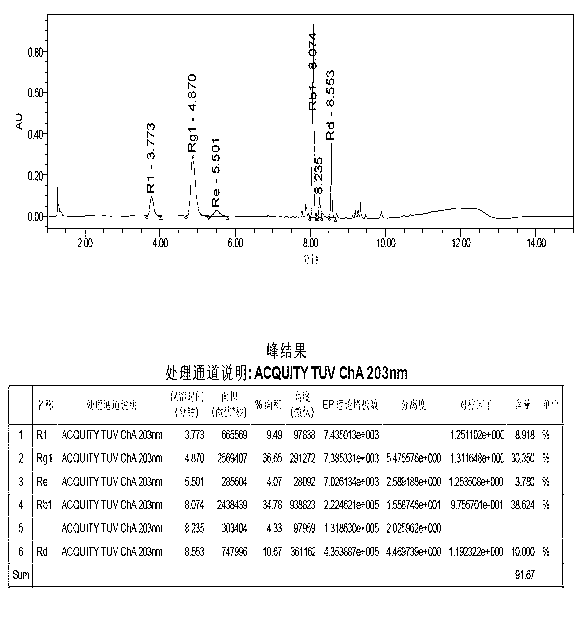
[0172] The method of the present invention is applied to five kinds of saponins (notoginseng saponin R 1 , Ginsenoside Rg 1 , Ginsenoside Re, Ginsenoside Rb 1 , Ginsenoside Rd) content determination.
[0173] (1) Collect 100 batches of dispersible tablets prepared from Panax notoginseng extract, 72 batches were randomly selected as calibration sample sets for modeling, and the remaining 28 batches were used as verification sample sets to verify the established calibration model.
[0174] (2) Collecting the near-infrared spectrum of each sample in the calibration sample set and the near-infrared spectrum of each sample in the verification sample set described in step (1) respectively are the original spectrum of the calibration sample set and the original spectrum of the verification sample set;
[0175] Scanning conditions: use the diffuse reflectance sample accessory of the integrating sphere. Before scanning the sample, scan the built-in background according to the scanni...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com