Preparation of fibroblast growth factor-1 modified gel and its application in the treatment of diabetic foot
A fibroblast and growth factor technology, applied in the field of biomedicine, can solve the problems of reducing the quality of life of patients, reducing the ability to resist infection, and immune dysfunction, etc., and achieve the effects of increasing clinical application, promoting effective healing, and delaying release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 production loading FGF-1 135 gel
[0025] Substrate swelling and sterilization: Add glycerin to Carbomer 940, stir and mix well, fully swell with appropriate amount of water for injection, adjust the pH value to form a transparent gel, and autoclave at 121°C for 30 minutes. Ready to use after cooling;
[0026] Protein sterilization and filtration: add recombinant human fibroblast growth factor-1 modified protein, human serum albumin and heparin into cooled water for injection, mix well, and filter with a 0.22 μm filter membrane to sterilize under sterile conditions ;
[0027] Gel preparation: under aseptic conditions, fully mix the above two substances, and divide into packages.
[0028] As a matrix material, carbomer has good ductility, is easy to apply and adhere to the skin, is non-irritating to the skin and mucous membranes, and can absorb tissue infiltration fluid, which is conducive to the discharge of secretions; it is non-greasy, releases drugs quic...
Embodiment 2
[0029] Embodiment 2 measures gel activity
[0030]The activity of the gel was determined by tetramethylazolium salt colorimetric method (MTT method). Balb / c3T3 cells were cultured in complete medium at 37°C and 5% CO2 until logarithmic growth phase. Discard the culture medium in the culture flask, digest and collect the cells, add complete medium to adjust the concentration of the cell suspension, so that the density of the cells to be tested is 50,000-100,000 / ml, inoculate 100 μl per well in a 96-well cell culture plate, Cultivate at 37°C and 5% CO2 for 24 hours, then change the maintenance medium and continue to cultivate for 24 hours. Discard the maintenance solution, add the standard substance and the gel sample solution dissolved in the medium and diluted at a certain concentration, 100 μl per well, and incubate in a 37°C, 5% CO2 incubator for 64h to 72h. Add 20 μl of MTT solution to each well and continue to incubate for 5 h. Pour off the liquid, add 100 μl dimethyl s...
Embodiment 3
[0031] Embodiment 3 measures the stability of gel
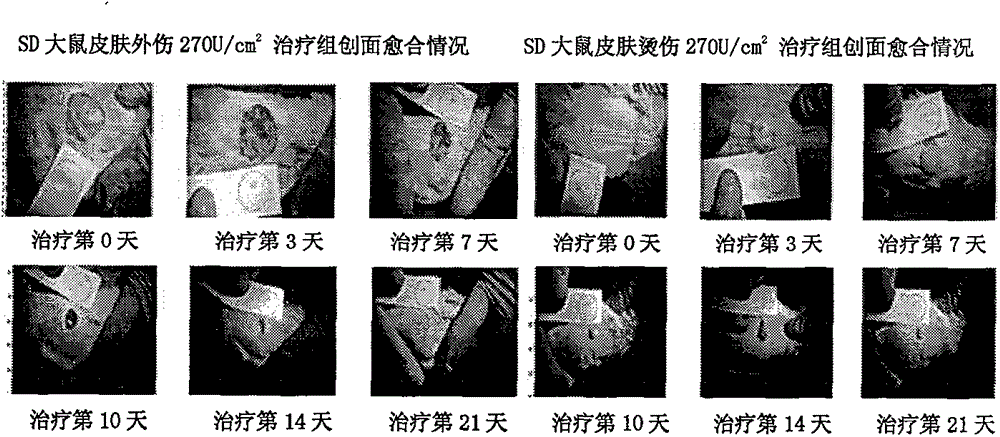
[0032] According to the test results of the stability of the gel at different temperatures, the gel has no mildew and its appearance, pH value and other physical and chemical indicators have not changed after being stored at 4°C, 25°C, and 37°C for 12 months. ; The biological activity of the drug-loaded gel has poor stability at room temperature, and the activity is significantly reduced after being placed for 3 months, while low temperature conditions have no obvious impact on the storage of the drug-loaded gel.
[0033] Table 1FGF-1 gel at 25 ℃ stability test result
[0034]
[0035] Table 2FGF-1 gel stability test results at 4°C
[0036]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com