Conjugate of influenza A virus conservative peptides M2e and virus-like particles, and application thereof
A type A influenza virus, virus-like technology, applied in the direction of antiviral agents, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve problems such as no good protective effect and dependence on chicken embryos , to achieve the effect of easy large-scale production and short preparation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1. Preparation of M2e-Q β-VLP conjugate and reference substance M2e-keyhole limpet hemocyanin (KLH) conjugate
[0019] Step 1. Preparation of bacteriophage virus-like particle Q β-VLP: inoculate the strain Escherichia coli DH5α / pETQ β-CP into LB medium and cultivate until the appropriate bacterial solution reaches the OD value, add the inducer IPTG to induce for several hours, and collect the bacteria Cells were disrupted by ultrasound, and after lysis, the supernatant and pellet were collected by centrifugation. The formed multimer was purified using Sepharose CL-4B chromatography column.
[0020] Step 2, the synthesis of M2e polypeptide: adopt the solid-phase synthesis method to utilize the polypeptide synthesizer to automatically synthesize the conserved sequence M2e of human influenza A virus M2 protein
[0021] Step 3. The peptide segment M2e is coupled to the bacteriophage virus-like particle Q β-VLP and keyhole limpet hemocyanin (KLH) respectively.
[0...
Embodiment 2
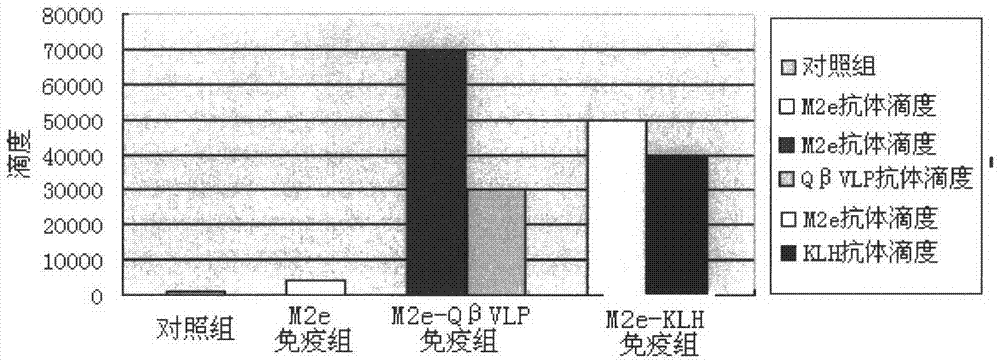
[0023] Embodiment two, ELISA method detection mouse serum antibody titer
[0024] Twenty 6-week-old C57BL / 6 female mice were randomly divided into 4 groups, with an average of 5 mice in each group. The groups were divided into M2e polypeptide group, M2e-QβVLP group, M2e-KLH group and control group. See Table 1 below for the immunization methods, doses, time intervals, and times.
[0025] Table 1: Immunization strategy
[0026] time
times
dosage
Immunization method
0 weeks
1st
50ug
subcutaneous more
2 weeks
2nd
50ug
subcutaneous more
4 weeks
the 3rd time
50ug
subcutaneous more
[0027] 100ul of mouse serum was collected before each immunization. Store at -20°C.
[0028] Dissolve M2e polypeptide, VLP, and KLH in 0.1mM carbonate buffer solution (pH 9.6), make 20ug / mL antigen coating solution, coat 96-well plates with 100ul per well, and coat overnight at 4°C. quilt. Then it was blocke...
Embodiment 3
[0030] Embodiment 3, mouse protective experiment
[0031] 56 8-week-old C57BL / 6 female mice were randomly divided into 4 groups, with an average of 14 in each group. The groups were divided into placebo group, M2e-Q βVLP group, M2e-KLH group, and M2e group. See Table 2 below for the immunization methods, doses, time intervals, and times.
[0032] Table 2: Immunization schedule for C57BL / 6 mice
[0033] time
[0034] On the 28th day, 7 of the mice in each group were inoculated with 4 times the semi-lethal dose of influenza A strain (A / PR / 8 / 34[PR8, H1N1] PR8 strain, and the other 7 mice were inoculated with 4 times the semi-lethal dose of Influenza A virus NIB-64A / Perth / 16 / 2009[H3N2] strain, followed by closely observing the survival status of the mice every day, continued to observe for 28 days. The number of survival mice was counted. The experimental results are shown in Table 3. The experimental results show that, The M2e-QβVLP group was immunized twice with 50...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com