Spirofluorene derivative and preparation method and application thereof
The technology of a derivative and spirofluorene is applied in the field of spirofluorene derivatives and the preparation thereof, and achieves the effects of satisfying use requirements, easy preparation method and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
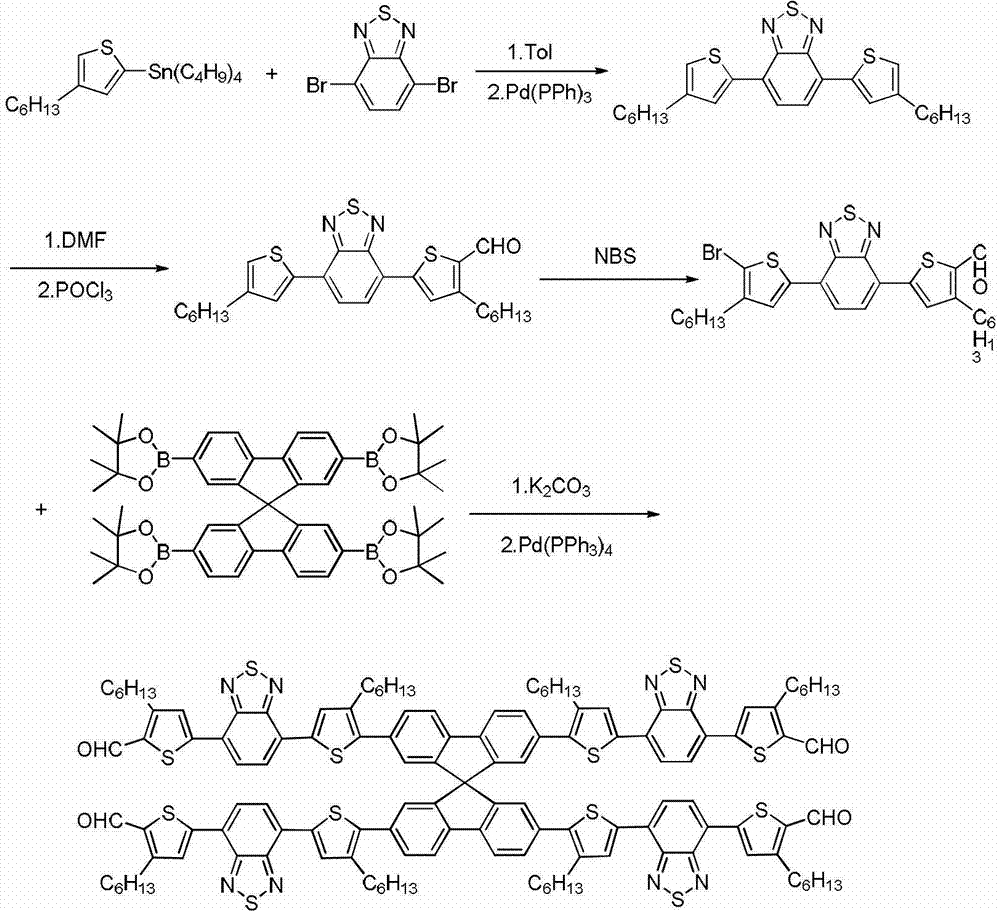
[0064] Synthesis of Compound II
[0065] Add 4,7-dibromobenzothiadiazole (8.82g, 30mmol), 4-hexyl-2-tributyltinthiophene (34.29g, 75mmol) in a 200ml single-necked bottle, N 2 Under protection, adding Pd(PPh 3 ) 4 (347mg, 0.3mmol), injected into 110mL of toluene, refluxed at 115°C for 24h. Stop the reaction, cool to room temperature, add 150mL water, extract three times with chloroform, combine the organic phases, and use anhydrous Na 2 SO 4 Dry, filter, and spin-dry to obtain a crude product, which is separated by silica gel column chromatography (trichloromethane / petroleum ether=1:3 as eluent) to obtain compound II (11.67 g, 83%).
[0066] Its structure is shown in the following formula:
[0067]
Embodiment 2
[0069] Synthesis of compound III
[0070] In a 250ml two-necked round bottom bottle, add compound II (5.63g, 12mmol), N 2 Inject 100mL of 1,2-dichloroethane under protection, N',N'-dimethylformamide (0.94mL, 12.6mmol), inject phosphorus oxychloride (1.10mL, 12.6mmol) at 0°C, and reflux Reaction 12h. Stop the reaction, cool to room temperature, pour the reaction solution into 100mL 2M sodium acetate aqueous solution, stir for 2h, extract with chloroform, wash twice with water, and wash with anhydrous Na 2 SO 4 Dry, filter, and spin-dry to obtain a crude product, which is separated by silica gel column chromatography (trichloromethane / petroleum ether=1:1 as eluent) to obtain compound III (5.00 g, 84%).
[0071] Its structural formula is as follows:
[0072]
Embodiment 3
[0074] Synthesis of Compound IV
[0075] In a 150 mL single-necked bottle, compound III (4.52 g, 9.1 mmol) was dissolved in 100 mL of chloroform, and NBS (1.72 g, 9.56 mmol) was added to the reaction solution five times, and reacted at room temperature for 12 h in the dark. Pour the reaction solution into 200mL water, extract with chloroform, combine the organic phases, and use anhydrous Na 2 SO 4 Dried, filtered, and spin-dried to obtain a crude product, which was recrystallized from chloroform and ethanol (1:1) to obtain compound IV (5 g, 95%). 1 H NMR (400MHz, CDCl 3 ): δ10.11(s, 1H), 8.07(s, 1H), 7.97(d, 1H), 7.83(s, 1H), 7.80(d, 1H), 3.03(t, 2H), 2.64(t, 2H), 1.81 (m, 2H), 1.67 (m, 2H), 1.34-1.44 (m, 12H), 0.92 (m, 6H).
[0076] Its structural formula is as follows:
[0077]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com