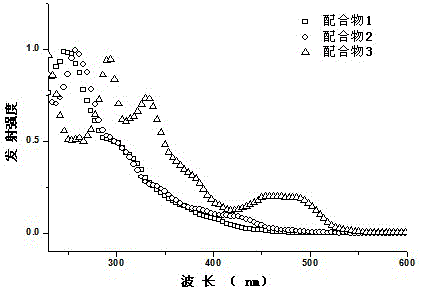
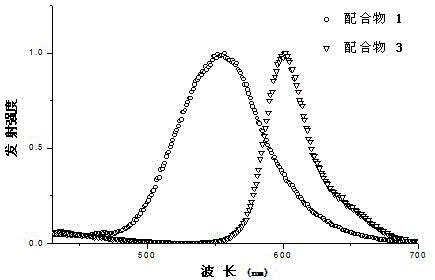
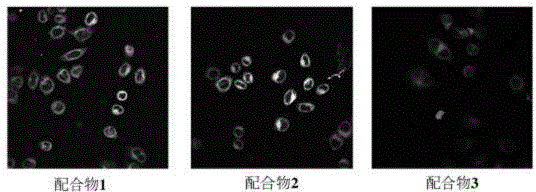
A class of boron-containing heteronuclear iridium complexes and their preparation methods and applications
An iridium complex and heteronuclear technology is applied in the field of preparation of new boron-containing heteronuclear iridium complexes, which can solve problems such as complex chemical reactions, limited development and application, and achieve simple synthesis steps, good cell staining effect, and mild conditions. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] The preparation of embodiment 1 iridium complex 1:
[0027] a. Synthesis of ligand (dfppy): difluorophenylboronic acid (5 mmol), Pd(PPh 3 ) 4 (4%) joins in the there-necked flask, vacuumizes-inflates nitrogen-vacuumizes on the double row pipe, circulates three times, finally protects the reaction system with nitrogen. Draw 2-bromopyridine (2 mL) into the reaction system with a syringe. Draw purified toluene (20mL), 2M potassium carbonate solution (10mL) and ethanol (10mL) into the reaction system with a syringe. Stir, and raise the temperature of the reaction system to 80°C, avoid light for reaction, and reflux for 24h. After the reaction, the solvent was removed, and DCM was used as the eluent, and the column was purified by column chromatography. Recrystallization (DCM / n-hexane) gave an oily product with a yield of 80%.
[0028]
[0029] b. at N 2 Under protective conditions, dissolve 1 equivalent of iridium trichloride and 2.2 equivalents of ligand (dfppy) i...
Embodiment 2
[0031] The preparation of embodiment 2 complex 2:
[0032] in N 2 Under protective conditions, dissolve 1 equivalent of iridium trichloride and 2.2 equivalents of ligand (ppy) in a mixture of 2-ethoxyethanol and water (3:1, v:v), and react under reflux for 24 hours ; After being cooled to room temperature, add a large amount of water, have precipitation to produce, suction filtration, dry under the infrared lamp to obtain the iridium dichloro bridge intermediate; 2 CO 3Dissolve in 2-ethoxyethanol, reflux and stir to react for 24 hours; after the reaction, suction filter, spin dry the filtrate under reduced pressure, add dichloromethane to dissolve, separate by column chromatography, and recrystallize to obtain the iridium complex intermediate; Then add boron trifluoride diethyl ether diluted with dichloromethane dropwise to the dichloromethane solution in which the iridium complex is dissolved, and stir at room temperature for 12 hours; after the reaction, the solvent is rem...
Embodiment 3
[0034] The preparation of embodiment 3 iridium complex 3:
[0035] a. Synthesis of ligand (thq): Add 2-nitrobenzaldehyde (3.8g, 25mmol), iron powder (10g, 178.5mmol), 85mL absolute ethanol, 85mL glacial acetic acid and 45mL deionized water into a three-necked flask , reflux reaction for half an hour. After the reaction was finished, filter with suction and collect the filtrate with CH 2 Cl 2 After extraction three times, the organic phase was washed three times with saturated aqueous sodium bicarbonate solution and deionized water, dried over anhydrous magnesium sulfate, and spin-dried to obtain anthranilaldehyde as a yellow oil. Yield 70%. Then, 5.9 g of diacetylthiophene, 2.8 g of NaOH, and 40 mL of ethanol were added to 5.7 g of anthranilaldehyde, and refluxed overnight. After the reaction was completed, it was cooled and filtered, and the obtained crude product was recrystallized from ethanol to obtain white needle-like crystals with a yield of 70%.
[0036]
[003...
PUM
| Property | Measurement | Unit |
|---|---|---|
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com