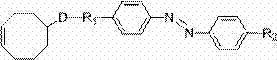
Side chained linear azobenzene liquid crystal polymer material and preparation method thereof
A technology of liquid crystal polymers and azobenzene, which is applied in the direction of liquid crystal materials, chemical instruments and methods, plastic recycling, etc., can solve the problems of insolubility and inability to melt, and achieve the effect of unlimited shape and good light response characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Selecting monomer C11AB6 to prepare side chain linear azobenzene liquid crystal polymer material PC11AB6
[0033]
[0034] (1) Synthesis of epoxy cyclooctene
[0035] Add 42 g of 1,5-cyclooctadiene to a two-neck round bottom flask, dissolve 90 g of m-chloroperoxybenzoic acid (70-75% by mass) in chloroform, and drop them into the flask. After the dropwise addition was completed, after the reaction was stirred at room temperature, the white precipitate was removed by filtration, the organic layer was washed with aqueous sodium bisulfite, aqueous sodium bicarbonate and aqueous sodium chloride, and distilled under reduced pressure to obtain epoxycyclooctene.
[0036] (2) Synthesis of 5-hydroxyl-1-cyclooctene
[0037] In the three-necked flask, add 50 mL of anhydrous tetrahydrofuran solution of 1 mol / L lithium aluminum hydride. After 10 g of epoxidized cyclooctene was dissolved in tetrahydrofuran, it was added dropwise into a three-necked flask under the prote...
Embodiment 2
[0052] Example 2 Selection of monomer C9AB to prepare side chain linear azobenzene liquid crystal polymer material PC9AB
[0053]
[0054] (1) Synthesis of 4'-cyano-4-hydroxyazobenzene
[0055] In a beaker, dissolve 5 g of 4-cyanoaniline in hydrochloric acid, add 20 mL of an aqueous solution of 3 g of sodium nitrite, stir at low temperature for 2 hours, add 4 g of phenol, 2 g of sodium hydroxide and 20 mL of water to form Aqueous solution, adding sodium carbonate solution dropwise to adjust the pH value, stirring at low temperature for 3 hours, adding hydrochloric acid dropwise to adjust the pH value to acidity, suction filtering to obtain a solid, and then recrystallizing with ethanol to obtain the product 4'-cyano-4-hydroxyazobenzene .
[0056] (2) Synthesis of 4'-(9-hydroxynonyloxy)-4-cyanoazobenzene
[0057] In a two-necked flask, dissolve 3 g of 4'-cyano-4-hydroxyazobenzene in 20 mL of DMF, add 2 g of potassium carbonate, then add 3 g of 9-bromo-1-nonanol, heat and s...
Embodiment 3
[0062] Example 3 Selecting monomer C12AB2 to prepare side-chain linear azobenzene liquid crystal polymer material PC12AB2
[0063]
[0064] (1) Synthesis of 4-ethoxy-4’-hydroxyazobenzene
[0065] 10 g of 4-ethoxyaniline was dissolved in 280 mL of hydrochloric acid (2 mol / L), added to a large beaker, cooled, and magnetically stirred. 5g NaNO 2 Soluble in water, slowly add dropwise to the above solution at low temperature to generate diazonium salt. Dissolve 6 g of phenol and 8 g of sodium hydroxide in water, and slowly add it dropwise to the above solution after cooling. After the dropwise addition was completed, the reaction was carried out at low temperature for 2 h. The pH was adjusted with hydrochloric acid, and the solid crude product was obtained by filtration. The crude product was separated by column chromatography with ethyl acetate and recrystallized from ethanol to obtain 4-ethoxy-4'-hydroxyazobenzene.
[0066] (2) 4-ethoxy-4'-(12-carboxydodecyloxy)azobenzene...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com