Functional polymer containing amino aryl ethylene and preparation method of functional polymer
A technology of aminoaromatic ethylene and aromatic vinyl, which is applied in the field of functional polymers of aminoaromatic ethylene and its preparation, can solve problems such as difficulty in removing o-chloroaniline, unfavorable environmental protection, long reaction cycle, etc., and achieve environmental protection Effects of industrial application, ease of industrial application, and raw material cost advantages
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
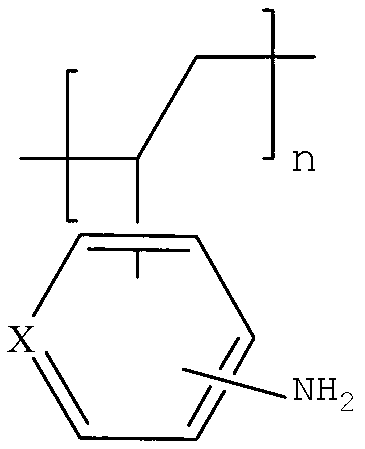
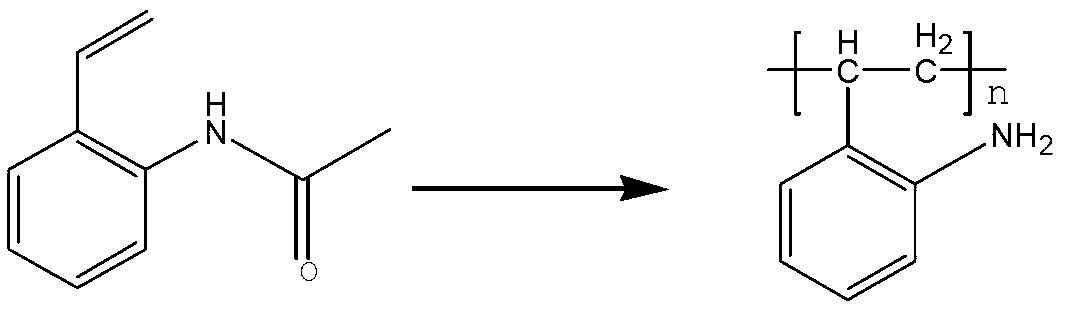
[0052]Add 400 grams of ethanol, 100 grams of deionized water, and 80.5 grams (0.5 moles) of 2-acetamidostyrene into a 1000 milliliter stainless steel reactor; 1 g of ethanol solution, after dropping, heat up to an internal temperature of 115°C to 120°C, react for 3 hours, and the pressure at this time is 4-5 atmospheres; cool to an internal temperature of 80-85°C, add 0.8 g of dodecyl mercaptan , heat preservation reaction for 2 hours; filter while hot to remove a small amount of high molecular weight polymer; cool the filtrate to 50 degrees, add 100 grams of 25wt% sodium hydroxide aqueous solution, heat to 95-100 ° C for 4 hours; cool to 20 ° C, Filtrate and dry to obtain 56.5 g of solid poly-2-aminostyrene formula (1). The appearance is off-white solid particles, insoluble in cold water, slightly soluble in hot water, soluble in hot alcohol, and the yield is 95.0%.
Embodiment 2
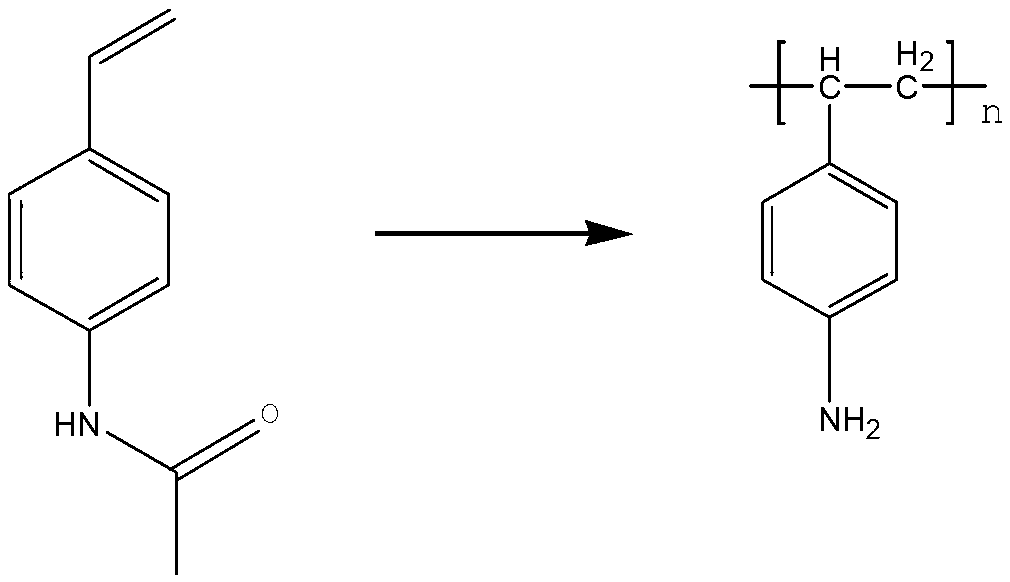
[0054] 80.5 grams of 4-acetamidostyrene was used as a monomer to replace 2-acetamidostyrene in Example 1 for free radical polymerization. The specific reaction process was the same as in Example 1. The polymer yield is shown in Table 1.
Embodiment 3
[0056] 80.5 grams of 3-acetamidostyrene was used as a monomer to replace 2-acetamidostyrene in Example 1 for free radical polymerization. The specific reaction process was the same as in Example 1. The polymer yield is shown in Table 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com