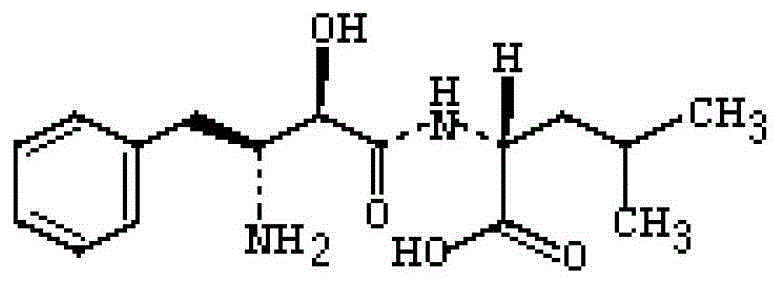
Ubenimex capsule composition and preparation method thereof
A technology of Ubenimex and composition, which is applied in the field of Ubenimex capsule composition and its preparation, can solve the problems of long-term stability investigation of uninjected liquid, influence of Ubenimex stability, complex preparation process, etc. To achieve the effect of improving bioavailability, cheap price of finished medicine, and overcoming dissolution and disintegration hysteresis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
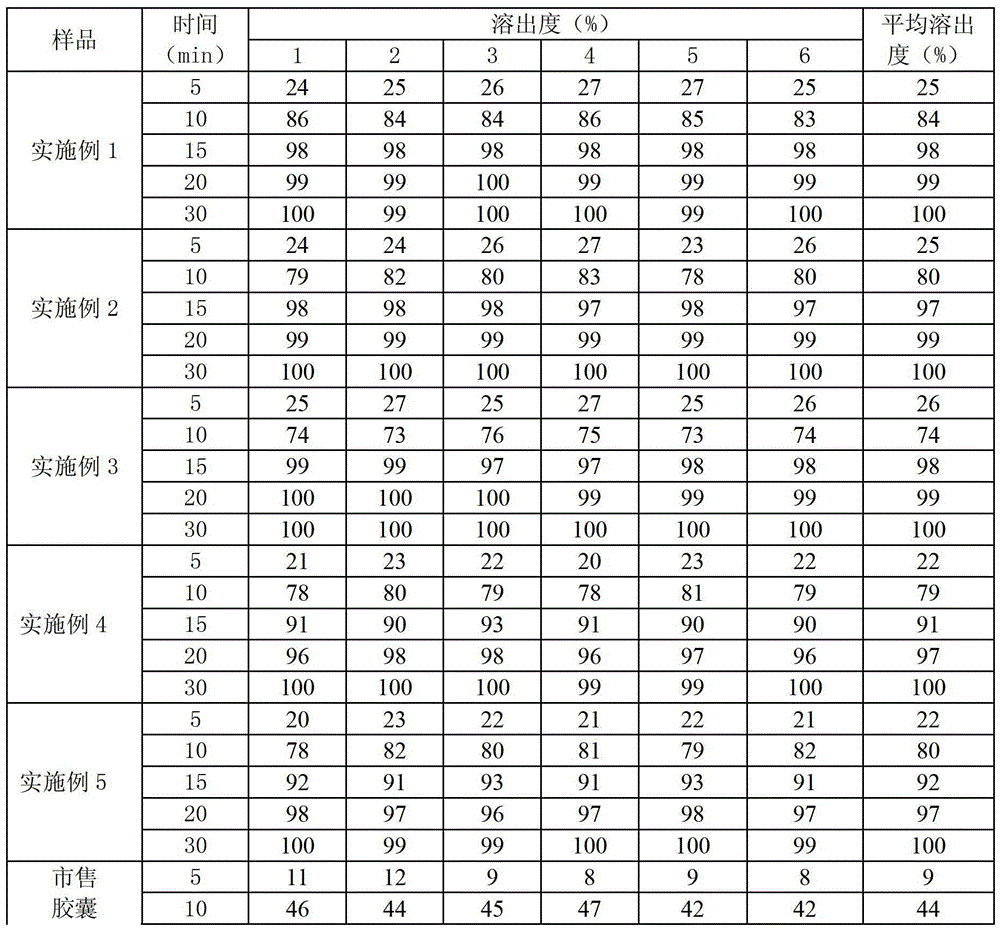
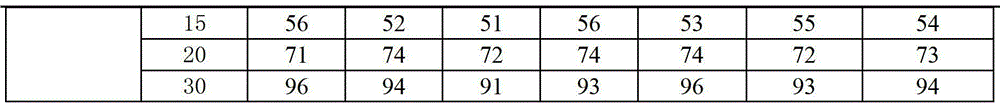
Examples
Embodiment 1
[0040] Preparation Process:
[0041] 1) Prepare the prescribed amount of povidone with 16% ethanol solution to make 12% PVP solution, then dissolve the prescribed amount of sodium lauryl sulfate and sucrose stearate in the solution, and finally add the prescribed amount Ubenimex, so that it can be dispersed evenly, and the drug-containing adhesive can be prepared;
[0042] 2) Pass the prescribed amount of lactose, microcrystalline cellulose, croscarmellose sodium and polacrilin potassium through 80-100 mesh sieves respectively, and mix them uniformly to prepare blank mixed auxiliary materials;
[0043] 3) Mix the drug-containing adhesive obtained in step 1) with the blank mixed auxiliary materials obtained in step 2 through the high-pressure sprayer of the spray dryer, and dry at 25°C for 5 minutes to obtain granules with a moisture content of 1.0%.
[0044] 4) Sizing the granules obtained in step 3) with an 18-mesh sieve;
[0045] 5) Add the granules obtained in step 4) to ...
Embodiment 2
[0047] The preparation process is the same as in Example 1, wherein the drying temperature in step 3 is 24°C, the drying time is 5 minutes, and the moisture content is reduced to 0.5%.
Embodiment 3
[0049] The preparation process is the same as in Example 1, wherein the drying temperature in step 3 is 23°C, the drying time is 4 minutes, and the moisture content is reduced to 1.3%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com