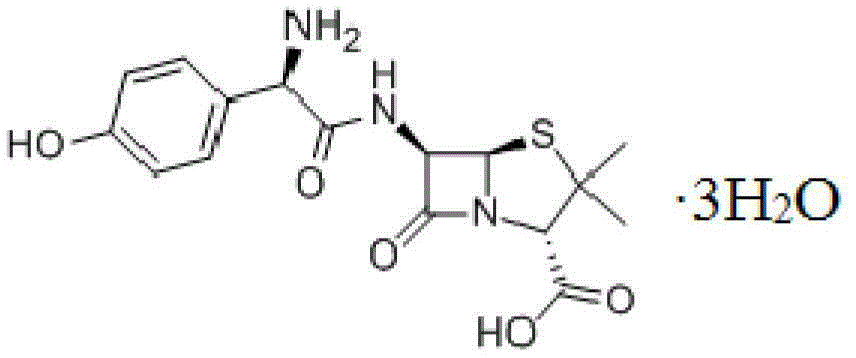
Amoxicillin compound and pharmaceutical composition of amoxicillin compound and potassium clavulanate
A technology of potassium clavulanate and amoxicillin, which is applied in the field of medicine, can solve the problems of poor drug dissolution in vitro, difficulty in industrial production, and influence on drug release, so as to improve stability and solubility, stabilize quality, and improve drug safety Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
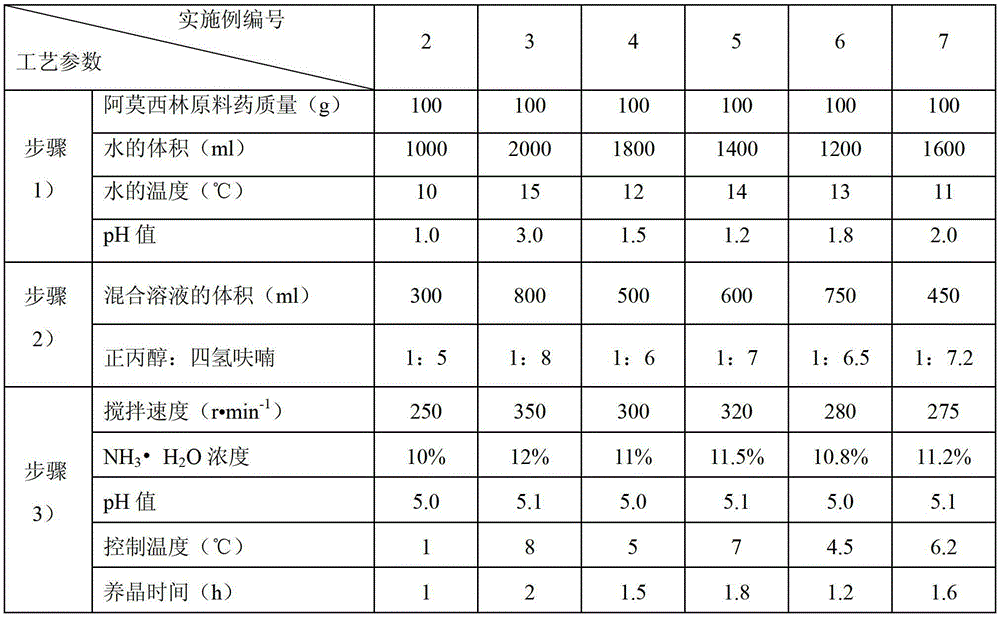
[0047] [Example 1] Preparation of Amoxicillin crystalline compound
[0048] (1) Add 100g of amoxicillin bulk drug to 1500ml of water at 12°C, adjust the pH value to 2.0 with hydrochloric acid, and obtain an aqueous solution of amoxicillin;
[0049] (2) Add 560ml of n-propanol / tetrahydrofuran mixed solution to the aqueous solution of amoxicillin, wherein the volume ratio of n-propanol to tetrahydrofuran is 1:6.2;
[0050] (3) After adding, the stirring speed is 275r·min -1 11% NH was added dropwise under stirring 3 ·H 2 O adjust the pH value to 5.0, precipitate crystals, control the temperature at 5°C, grow the crystals for 1.5 hours, then filter with suction, wash the filter cake with purified water and acetone in turn, and dry to obtain the crystalline amoxicillin compound.
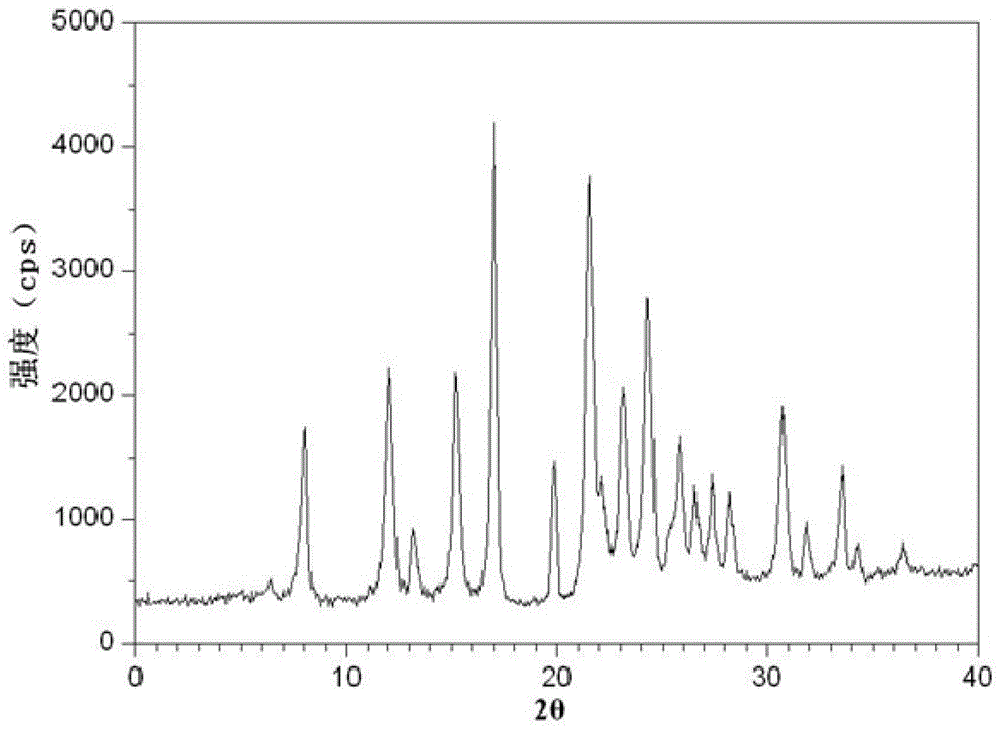
[0051] The characteristic peaks in the X-ray powder diffraction pattern of the obtained amoxicillin crystalline compound obtained by Cu-Kα ray measurement are 8.0°, 12.1°, 15.4°, 17.0°, 19.8°, 21.6°, ...
preparation Embodiment 1
[0056] [Preparation Example 1] Amoxicillin and Clavulanate Potassium Tablets
[0057] Specification: Amoxicillin / clavulanic acid 875mg / 125mg
[0058] prescription:
[0059]
[0060] Preparation:
[0061] Excipients Microcrystalline cellulose, croscarmellose sodium, and micropowder silica gel were dried at 80°C for 4 hours and passed through an 80-mesh sieve; magnesium stearate was dried at 60°C for 4 hours; passed through a No. 9 sieve; set aside. Take the amoxicillin crystalline compound of Example 1 and amoxicillin-clavulanate potassium 2:1 mixed powder at a ratio of 7:1 by weight, measure the content of the two components and calculate the feeding amount. Add microcrystalline cellulose (internal addition part) and magnesium stearate (internal addition part) according to the prescription ratio, mix well, and dry press on a dry extrusion granulator to form granules under the condition that the relative humidity is not higher than 33%. The granules are mixed evenly with ...
preparation Embodiment 2
[0062] [Preparation Example 2] Amoxicillin and Clavulanate Potassium Tablets
[0063] Specification: Amoxicillin / clavulanic acid 136mg / 34mg
[0064] prescription:
[0065]
[0066] Preparation:
[0067] (a) take the amoxicillin crystalline compound of embodiment 2 (in C 16 h 19 N 3 o 5 S) 136g, potassium clavulanate (in C 8 h 9 NO 5 Total) 34g, microcrystalline cellulose (P112) 32g, croscarmellose sodium (ADS) 2g, micropowder silica gel 2g, magnesium stearate 2g, a total of 205g;
[0068] (b) After amoxicillin is granulated, it is mixed with potassium clavulanate at a mass ratio of 4:1 to form the main ingredient.
[0069] (c) Add and mix the auxiliary materials micropowder silica gel, croscarmellose sodium (ADS) and microcrystalline cellulose in equal amounts, and then add the same amount of main ingredients into the mixer and mix for 36-70 minutes.
[0070] During the whole process, the moisture content of the intermediate mixed powder is controlled to be ≤9.0%,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com