Mixed crystal form ferric fluoride cathode material and preparation method thereof
A cathode material, iron fluoride technology, applied in battery electrodes, electrical components, circuits, etc., can solve the problems of poor electrochemical cycle performance and complex preparation process, achieve excellent electrochemical performance, good repeatability, and improve mass ratio. Effects of Capacity and Thermal Stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
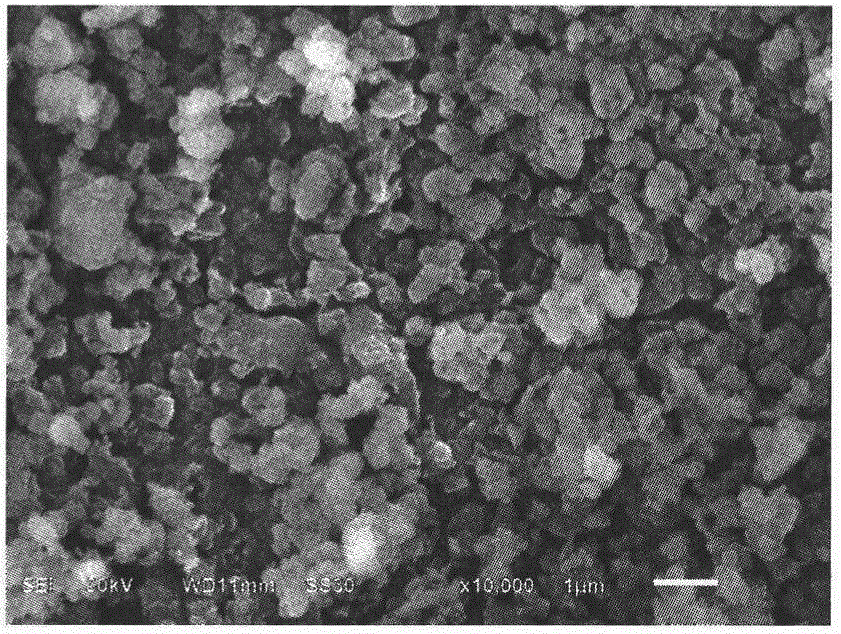
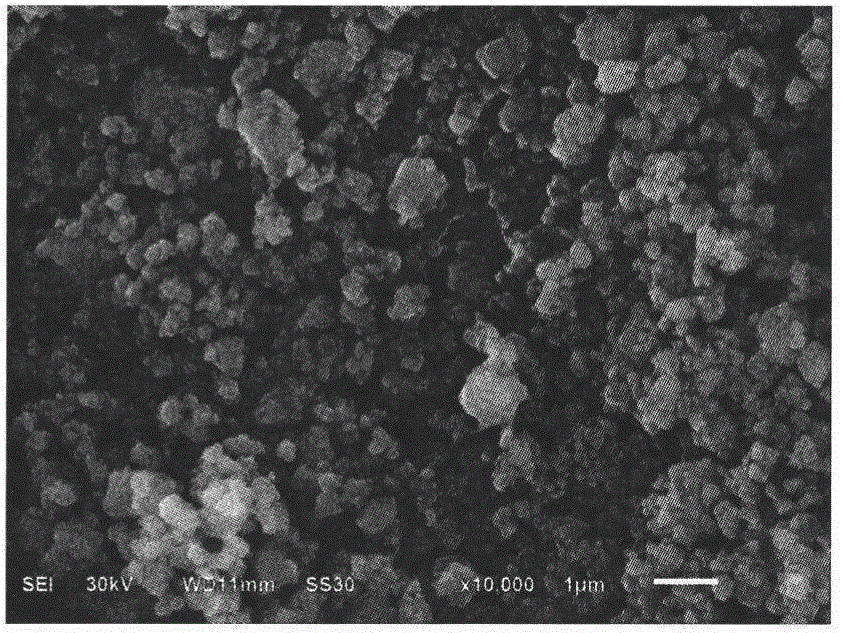
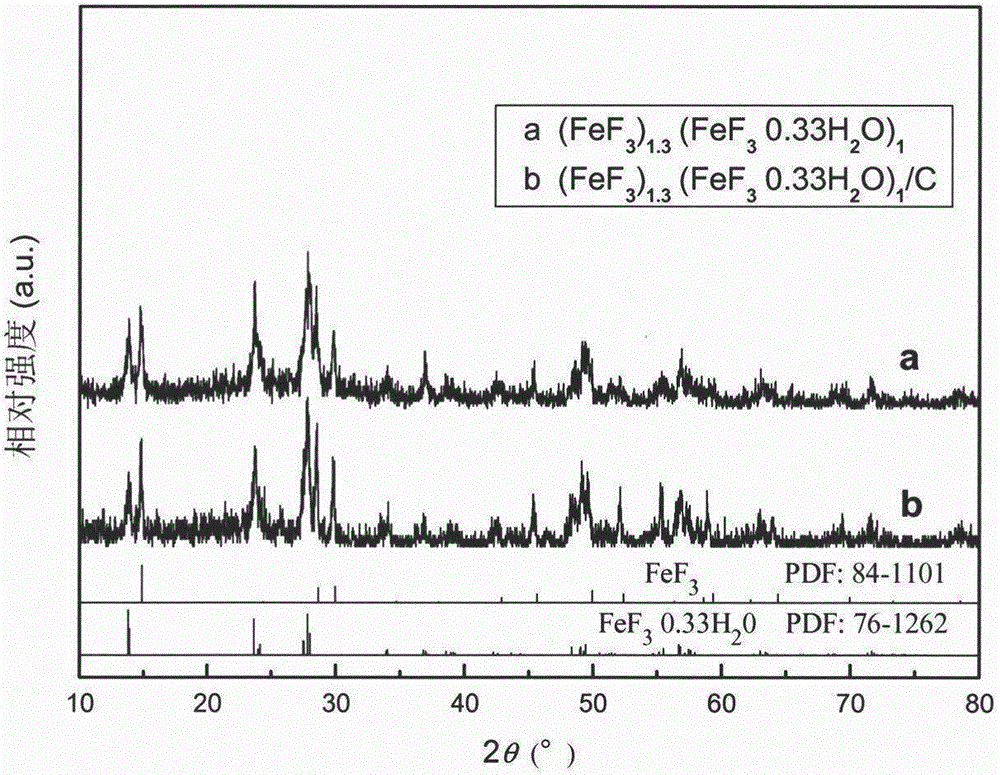
[0028] Weigh 4.8518g FeNO 3 9H 2 O, add 25mL of absolute ethanol, stir and dissolve, under normal temperature and constant stirring, slowly add 40wt% HF at a molar ratio of 1:4, put it into a sealed polytetrafluoroethylene container, stir at room temperature for 0.5h, and move to In a hydrothermal reaction kettle, conduct a hydrothermal reaction at 60°C for 24 hours to obtain a pink precipitate, pour off the supernatant, add 50mL of absolute ethanol, evaporate in an oil bath at 90°C until dry, and obtain (FeF 3 ) 1.3 (FeF 3 0.33H 2 O) 1 Powder. After it is mixed with 15wt.% acetylene black, it is placed in an agate ball mill jar, ball milled at a speed of 300r / min for 3h, and dried at 80°C for 12h in a drying oven to obtain (FeF 3 ) 1.3 (FeF 3 0.33H 2 O) 1 / C Composite.
Embodiment 2
[0030] Weigh 4.0432g FeNO 3 9H 2 O, add 25mL of absolute ethanol, stir and dissolve, at room temperature and under constant stirring, slowly add 20wt% NH 4 Put the mixed solution of ethanol and water into a sealed polytetrafluoroethylene container, stir at room temperature for 1.0h, move it to a hydrothermal reaction kettle, and conduct a hydrothermal reaction at 110°C for 6h to obtain a pink precipitate. Pour off the supernatant and add 50mL of absolute ethanol was evaporated at 90°C in an oil bath until dry to obtain (FeF 3 ) 2.1 (FeF 3 0.33H 2 O) 1 Powder. After it is mixed with 20wt.% acetylene black, it is placed in an agate ball mill jar, ball milled at a speed of 320r / min for 2h, and dried at 100°C for 10h in a drying oven to obtain (FeF 3 ) 2.1 (FeF 3 0.33H 2 O) 1 / C Composite.
Embodiment 3
[0032] Weigh 2.7038g FeCl 3 ·6H 2 O, add 25mL of absolute ethanol, stir and dissolve, at room temperature and under constant stirring, slowly add 20wt% NH 4 HF 2 Put the mixed solution of ethanol and water into a sealed polytetrafluoroethylene container, stir at room temperature for 0.5h, move it to a hydrothermal reaction kettle, and conduct a hydrothermal reaction at 100°C for 8h to obtain a pink precipitate. Pour off the supernatant and add 50mL Absolute ethanol was evaporated at 90°C in an oil bath until dry to obtain (FeF 3 ) 1.1 (FeF 3 0.33H 2 O) 1 Powder. After it is mixed with 10wt.% acetylene black, it is placed in an agate ball mill jar, ball milled at a speed of 280r / min for 4h, and dried at 90°C for 12h in a drying oven to obtain (FeF 3 ) 1.1 (FeF 3 0.33H 2 O) 1 / C Composite.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com