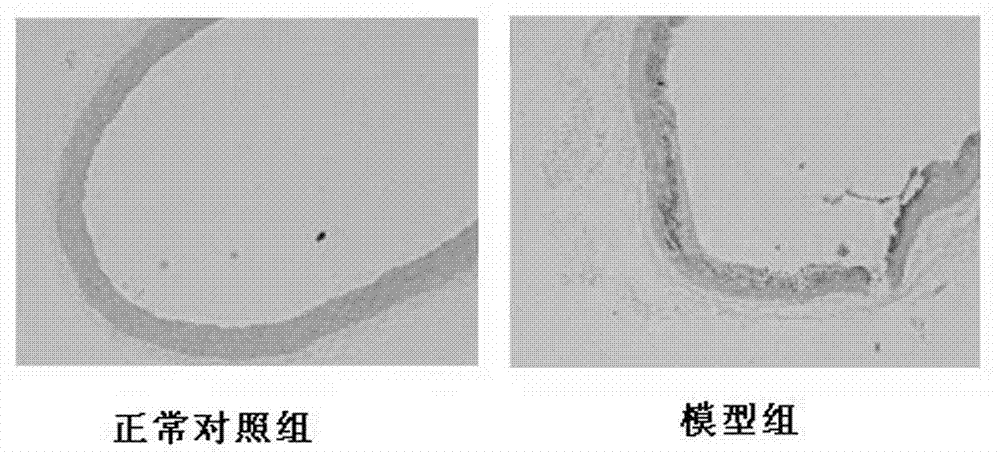
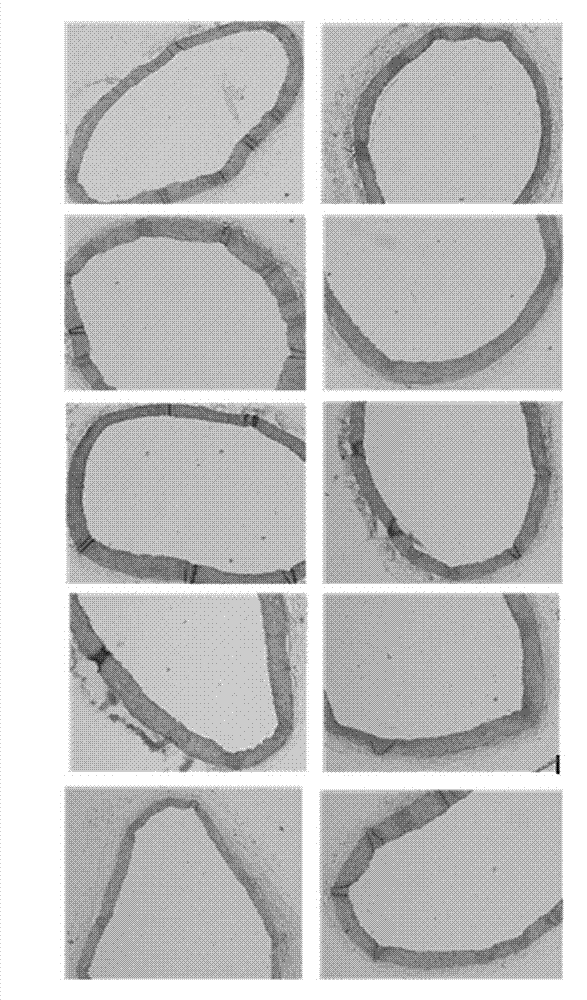
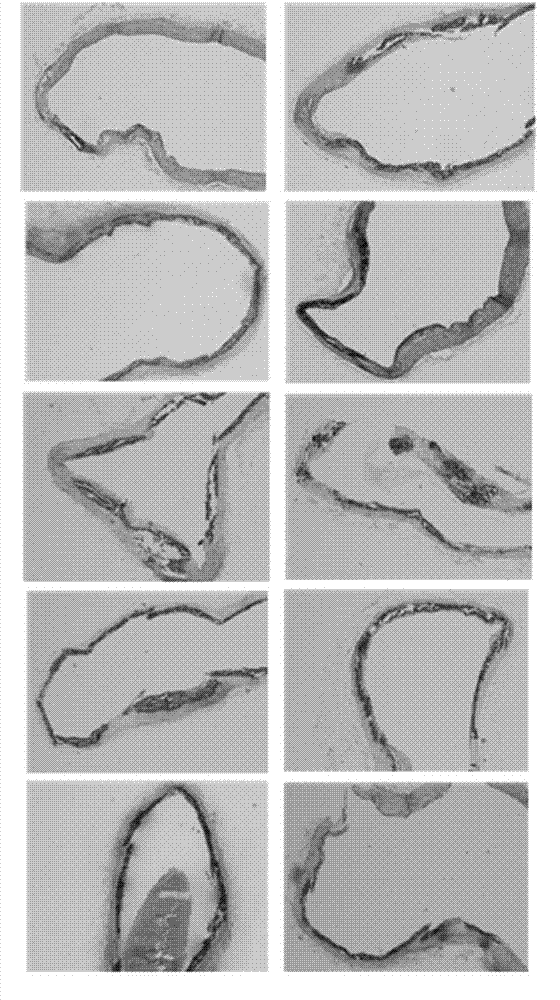
Application of polyhydroxy bromo-benzophenone compound and derivatives thereof in treatment and prevention of atherosclerosis
A technology for polyhydroxybromobenzophenone and hydroxybromobenzophenone is applied in the field of medicine, can solve problems such as few applications, and achieve the effects of simple preparation process, easy industrial synthesis and low price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of medicinal salt of 2,4',5'-trihydroxy-5,2'-dibromobenzophenone
[0031] Weigh 200mg of the sample into a 50mL flask, add 5mL of ethanol, stir and mix, add 20mL of 30% sodium hydroxide solution, stir in a 50°C water bath for 4 hours, add an appropriate amount of absolute ethanol, precipitate a white solid, and filter to obtain its sodium salt , the yield is 21.6%. HRMS (ESI): Calcd. for C 13 h 5 Br 2 Na 3 o 4 : 453.8224; Found: 453.8214.
[0032] .
[0033] Using the same method, 2,4',5'-trihydroxy-5,2'-dibromobenzophenone can react with other inorganic bases and organic bases to obtain its medicinal salts of inorganic bases and organic bases.
Embodiment 2
[0035] 2,4 ’ ,5 ’ -Trihydroxy-5,2 ’ -Preparation of dibromobenzophenone medicinal ester
[0036] Weigh 500mg of the sample into a 100mL flask, add 5g of succinic anhydride in 10mL of anhydrous dichloromethane solution, stir at room temperature, add 1.0g of DCC and 0.5g of DMAP in 20mL of anhydrous dichloromethane solution, stir at room temperature until the reaction of the raw materials is complete, slowly 10 mL of ice water was added to terminate the reaction, the insoluble matter was filtered off, and the organic phase was separated by silica gel chromatography to obtain white crystals with a yield of 45.3%. HRMS (ESI): Calcd. for C 25 h 20 Br 2 o 13 : 687.9336; Found: 687.9326.
[0037] .
[0038] 2,4 ’ ,5 ’ -Trihydroxy-5,2 ’ - Dibromobenzophenone can also react with acetyl chloride, propionyl chloride or acetic anhydride, propionic anhydride to obtain its pharmaceutically acceptable ester.
Embodiment 3
[0040] 2,4 ’ ,5 ’ -Trihydroxy-5,2 ’ -Preparation of dibromobenzophenone medicinal ether
[0041] Weigh 500 mg of sample and dissolve it in 20 mL of acetone, add a little potassium hydroxide, stir, add 10 mL of dimethyl sulfate dropwise, stir at room temperature, until the raw material reacts completely, remove acetone under reduced pressure, and the residue is washed with 10% sodium bicarbonate, After washing with distilled water, the crude product was separated and purified by silica gel chromatography to obtain white crystals with a yield of 37.8%. HRMS (ESI): Calcd. for C 16 h 14 Br 2 o 4 : 429.9227; Found: 429.9217.
[0042] .
[0043] Using the same method, 2,4 ’ ,5 ’ -Trihydroxy-5,2’ -Dibromobenzophenone can also react with diethyl sulfate to obtain its medicinal ether.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com