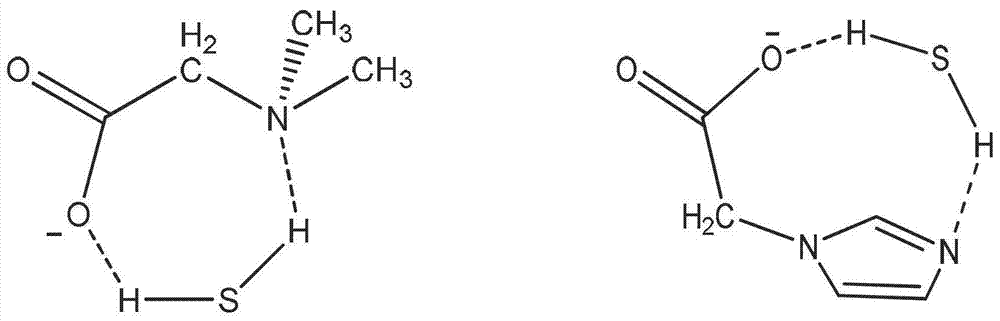
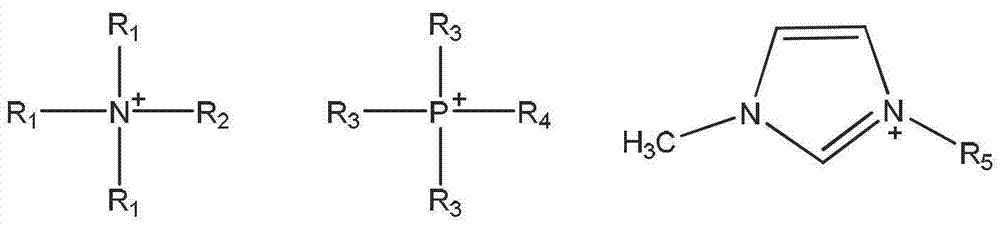
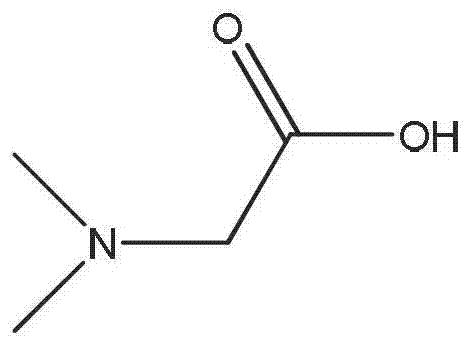
Ionic liquid compound with anion containing double Lewis base functional groups, preparation method thereof and application thereof
An ionic liquid and anion technology, which is applied in the preparation of organic compounds, compounds of group 5/15 elements of the periodic table, and cyanide reaction preparation, can solve the problems of low solubility and low absorption selectivity, and achieve high absorption capacity , good absorption selectivity and convenient synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1: the preparation of the ionic liquid that anion contains double weak Lewis basic functional group
[0039]Add triethylamine, bromo-n-butane and a certain amount of solvent ethanol in equimolar ratio respectively to the round-bottomed flask, reflux reaction at 80°C for 24 hours, after the reaction, the mixture is vacuum-dried at 60°C for 24 hours to Evaporate the solvent and unreacted raw materials to obtain N,N,N-triethylbutylammonium bromide. Dissolve N,N,N-triethylbutylammonium bromide in a certain amount of 50% (v / v) ethanol / water mixed solution, then add stoichiometric silver oxide in batches, and react for 6 hours at room temperature in the dark , and then filtered to remove the silver bromide formed in the reaction to obtain N,N,N-triethylbutylammonium hydroxide solution. It is also possible to make N,N,N-triethylbutylammonium bromide into an aqueous solution and mix with OH - Type anion exchange resin for anion exchange to obtain N,N,N-triethylbuty...
Embodiment 2
[0042] Embodiment 2: the preparation of the ionic liquid that anion contains double weak Lewis basic functional group
[0043] Using a method similar to Example 1, using triethylamine, bromo-n-butane and imidazole-1-acetic acid as raw materials, the ionic liquid triethyl-butylammonium imidazole-1 containing double weak Lewis basic functional groups can be obtained. 1-Acetate. Its chemical structural formula is:
[0044]
[0045] Characterization results: Elemental analysis theoretical value C62.42%, H10.10%, N15.60%, measured value C62.24%, H10.16%, N15.48%; H NMR spectrum 1 HNMR (300MHz, CDCl 3 ,25℃,TMS),δ:0.85ppm(3H,t),1.14ppm(9H,t),1.25ppm(2H,m),1.46ppm(2H,m),2.98ppm(2H,t),3.16 ppm(6H,q), 4.36ppm(2H,s), 6.80ppm(1H,d), 6.85ppm(1H,d), 7.37ppm(1H,s).
Embodiment 3
[0046] Embodiment 3: the preparation of the ionic liquid that anion contains double weak Lewis basic functional group
[0047] Using a method similar to Example 1, using triethylamine, bromo-n-butane and nicotinic acid as raw materials, the ionic liquid triethyl-butylammonium nicotinate containing double weak Lewis basic functional groups can be obtained. Its chemical structural formula is:
[0048]
[0049] Characterization results: Elemental analysis theoretical values C68.53%, H10.07%, N9.99%, Found: C68.46%, H10.12%, N9.89%; H NMR spectrum 1 HNMR (300MHz, CDCl 3 ,25℃,TMS),δ:0.80ppm(3H,t),1.17ppm(9H,t),1.24ppm(2H,m),1.46ppm(2H,m),3.04ppm(2H,t),3.26 ppm(6H,q), 7.11ppm(1H,t), 8.21ppm(1H,d), 8.39ppm(1H,d), 9.10ppm(1H,s).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com