Ilaprazole crystal form and preparation method thereof
A technology of ilaprazole crystal and crystal form, applied in the field of ilaprazole crystal form and preparation thereof, can solve the problems of poor stability, low efficacy and the like, and achieve the effects of good stability, easy preparation and low impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
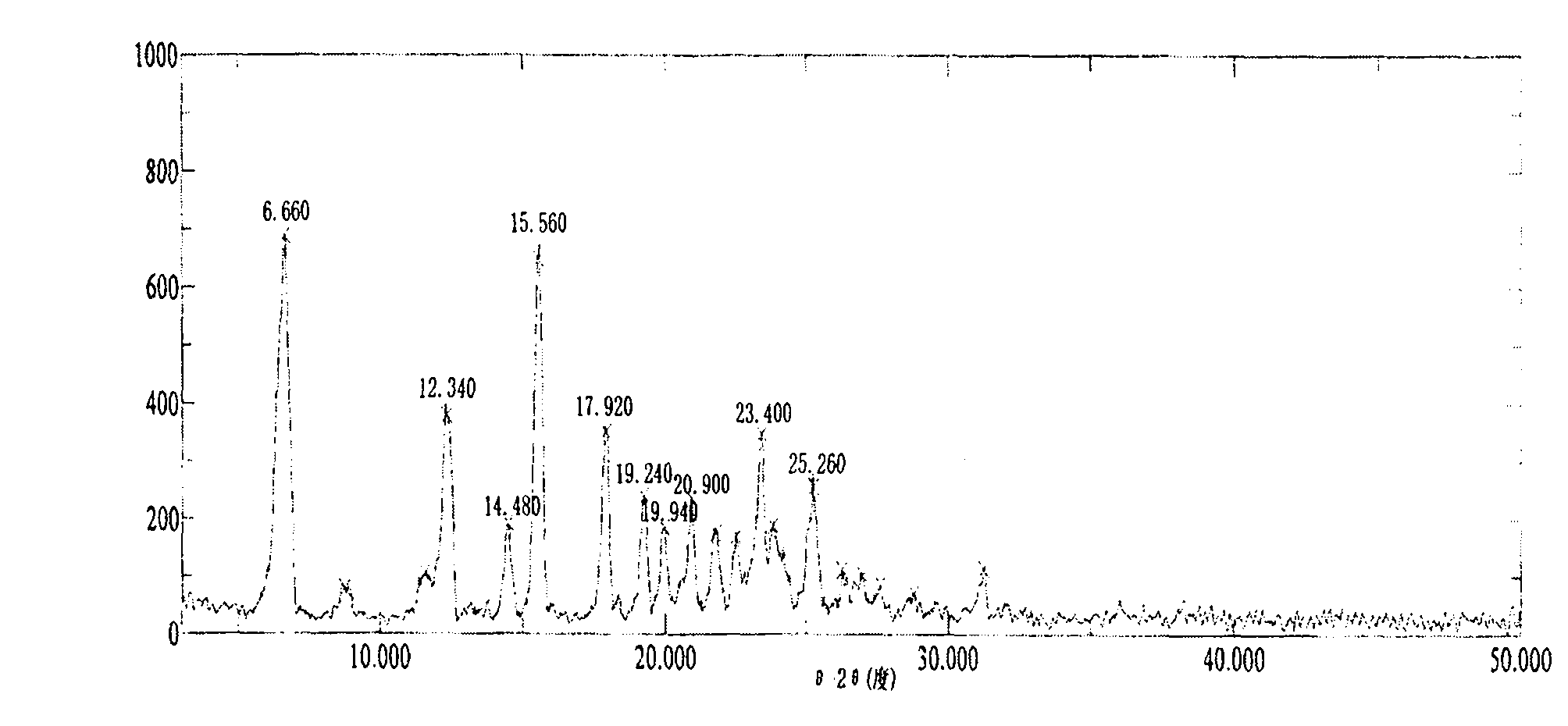
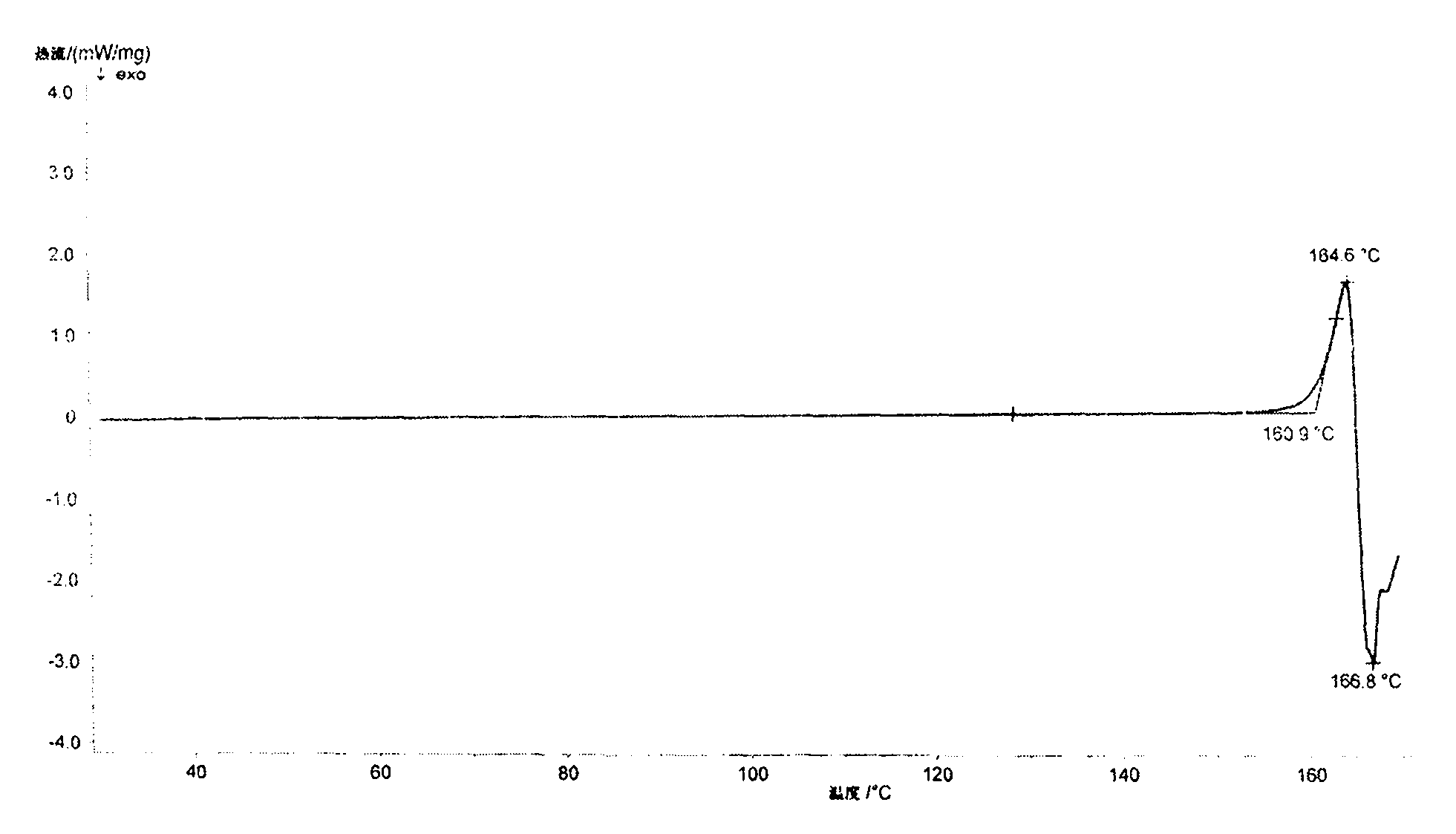
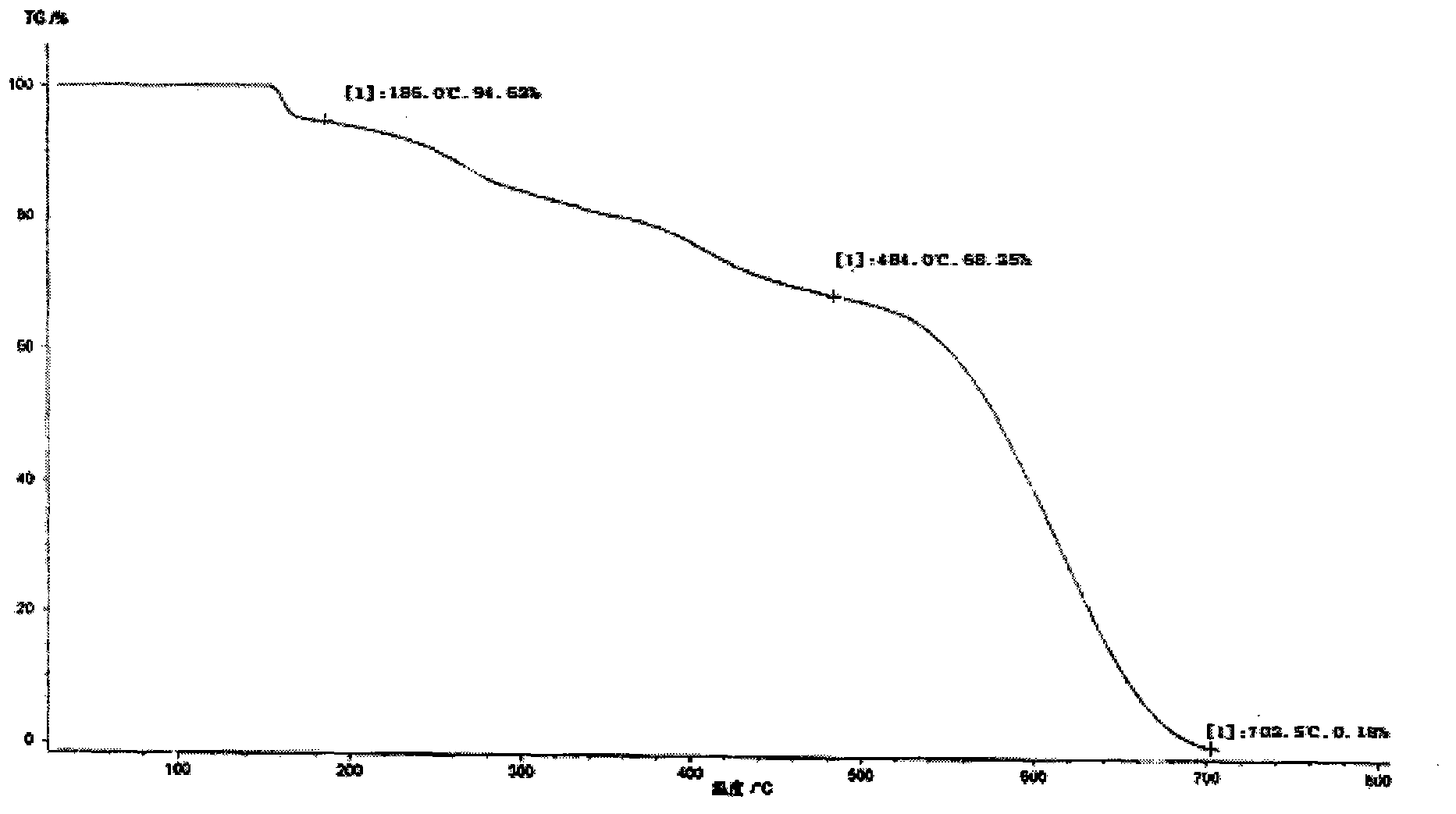
[0035] At 25°C, add 10g of ilaprazole to a 250ml single-necked flask, add 100ml of dichloromethane and 100ml of anhydrous methanol successively, stir the solution until the ilaprazole is completely dissolved, and then transfer the solution to a constant pressure funnel. The above-mentioned ilaprazole mixed solution is slowly added dropwise to 250ml of ether, and the temperature of the reaction system is controlled within the range of 25±2°C during the dropping process, and the dropping time is controlled between 25 and 30 minutes. After the addition was completed, the temperature was kept at 25°C and the solution was stirred for 40 minutes to crystallize. Vacuum filtration under reduced pressure, washing the filter cake with 150 ml of ether, and vacuum drying at room temperature for 24 hours to obtain an off-white powder, ilaprazole crystal form X, with a yield of 85%.
[0036] The Bruker D8Advance diffractometer was used to determine the X-ray powder diffraction pattern of ilapr...
Embodiment 2
[0047] At 25° C., 1 kg of ilaprazole was added to the reaction tank, 10L of dichloromethane and 10L of anhydrous methanol were added in sequence, the solution was stirred until the ilaprazole was completely dissolved, and then the solution was transferred to the metering tank. The above-mentioned ilaprazole mixed solution is slowly added to 25L of ether, and the temperature of the reaction system is controlled within the range of 25±2°C during the dropping process, and the dropping time is controlled between 25-30 min. After the addition was completed, the solution was stirred at 25°C for 40 minutes to crystallize. Vacuum filtration was performed under reduced pressure, the filter cake was washed with 15 L of ether, and dried under vacuum at room temperature for 24 hours to obtain an off-white powder of crystal form X with a yield of 88.8%.
[0048] After testing, the analysis result of ilaprazole crystal form X prepared in Example 2 is not significantly different from the analys...
Embodiment 3
[0050] At 25°C, add 10g of ilaprazole to a 250ml single-necked flask, add 100ml of chloroform and 100ml of absolute ethanol in sequence, stir the solution until the ilaprazole is completely dissolved, and then transfer the solution to a constant pressure funnel. The above-mentioned ilaprazole mixed solution is slowly added dropwise to 250ml of ether, and the temperature of the reaction system is controlled within the range of 25±2°C during the dropping process, and the dropping time is controlled between 25 and 30 minutes. After the addition was completed, the solution was stirred at 25°C for 40 minutes to crystallize. Vacuum filtration was performed under reduced pressure, the filter cake was washed with 150 ml of ether, and dried under vacuum at room temperature for 24 hours to obtain an off-white powder of crystal form X with a yield of 87%.
[0051] After testing, the analysis result of ilaprazole crystal form X prepared in Example 3 is not significantly different from the an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com