Method for preparing n-butanol from ethanol by using hydrothermal technique
A technology of n-butanol and ethanol, which is applied in the field of synthesizing n-butanol by hydrothermal technology, can solve the problems of high reaction temperature, high cost, and difficult operation, and achieve the effects of mild reaction conditions, low equipment requirements, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Put 0.295g (0.005mol) of metal cobalt powder, 0.84g (0.01mol) of sodium bicarbonate, 8.76mL (0.15mol) of absolute ethanol and 1.242mL (0.069mol) of distilled water into a 30mL autoclave to make The filling degree reaches 33%, react at 240°C for 3 days, and filter after the reactor is cooled.
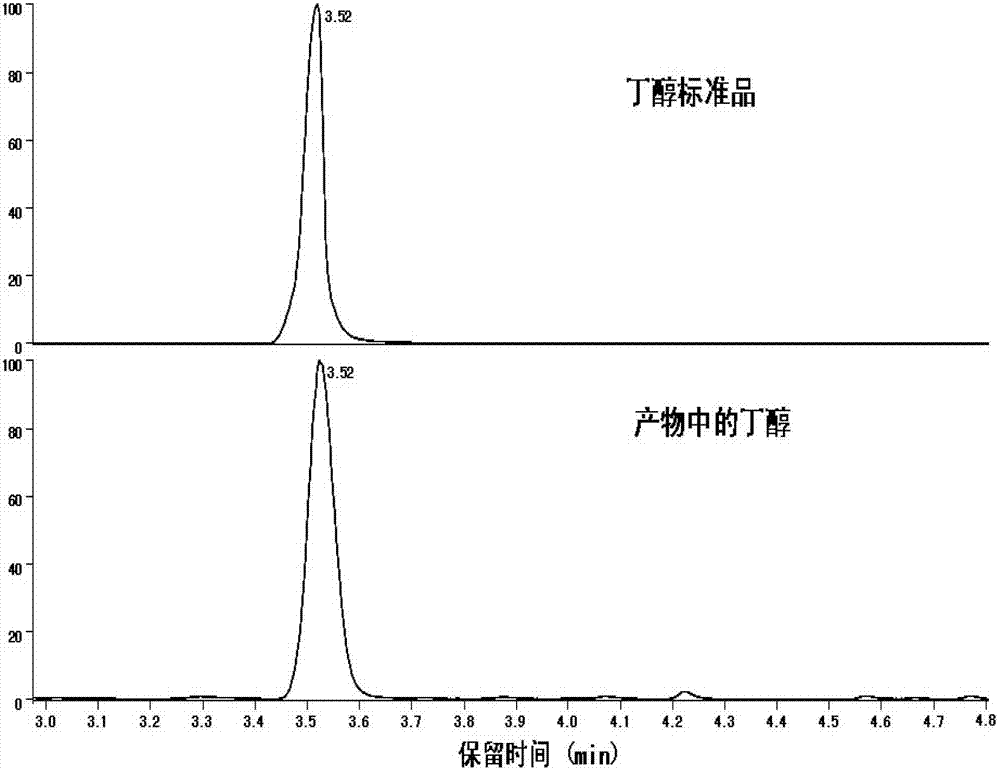
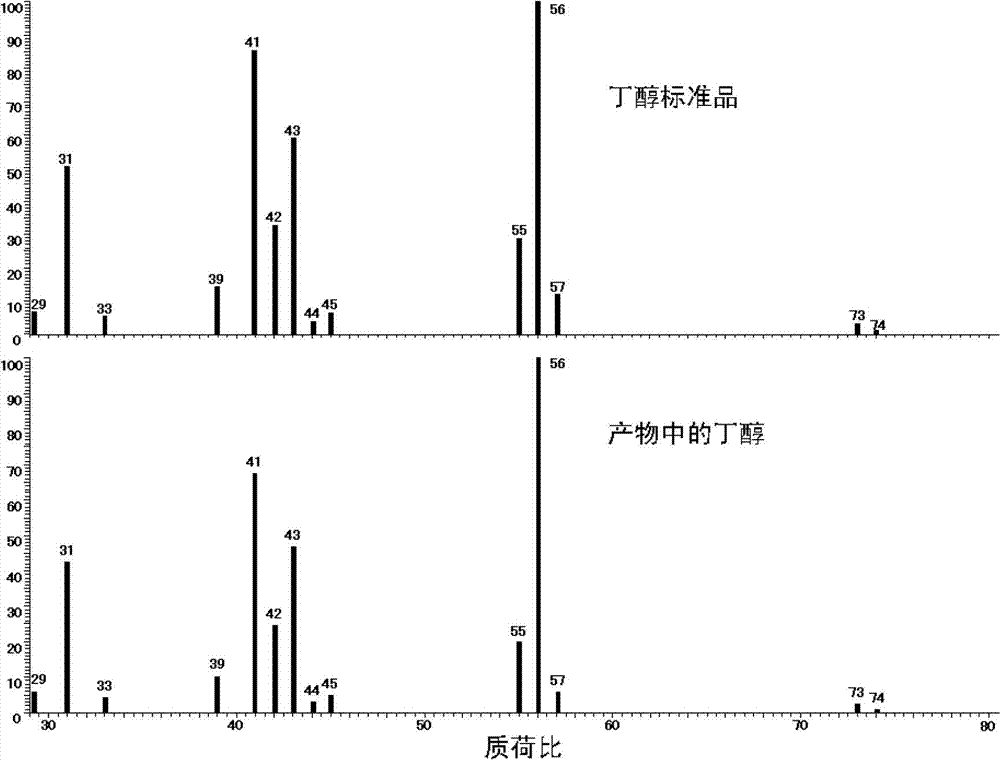
[0019] The obtained solution was detected by gas chromatography-mass spectrometry (TRACE DSQ GC-MS) (the column model was TR-wax-ms, the temperature of the injection port was 200°C, the temperature of the ion source was 250°C, the temperature of the transfer line was 250°C, and the sample injection The volume is 0.4μL, the split ratio is 10, the initial temperature of the programmed temperature rise is 80°C, and it is kept for 1 minute. The heating rate is 15°C / min, and the temperature is raised to 200°C, and it is kept for 6 minutes. mL / min). Compared with the retention time of the butanol standard sample chromatogram and the molecular ion peak and each fragment peak of the mass...
Embodiment 2
[0021] Add 0.295g (0.005mol) of metal cobalt powder, 0.84g (0.01mol) of sodium bicarbonate, 8.76mL (0.15mol) of absolute ethanol and 11.24mL (0.624mol) of distilled water into a 30mL autoclave to fill the The temperature reached 67%, reacted at 200°C for 30 days, and filtered after the reactor was cooled.
[0022] Gained solution is detected by gas chromatography-mass spectrometry (GC-MS), and according to the concentration-peak area standard curve and the product peak area, the concentration of n-butanol in the product of Example 2 can be calculated to be 0.36mol / L, selectivity 72%.
Embodiment 3
[0024] Add 0.42g (0.005mol) of sodium bicarbonate, 23.35mL of absolute ethanol (0.40mol) and 5.15mL (0.286mol) of distilled water into a 30mL autoclave, so that the filling degree reaches 95%, and react at 140°C for 30 Days, after the reaction kettle was cooled, it was filtered and the product was a colorless transparent liquid.
[0025] The resulting solution was detected by gas chromatography-mass spectrometry (GC-MS). No cobalt powder was added in Example 3, and n-butanol could be reacted to synthesize n-butanol, but the yield of n-butanol was low and the selectivity was low.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com