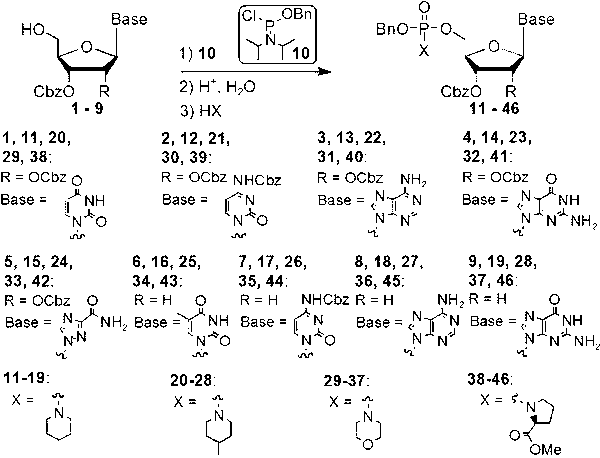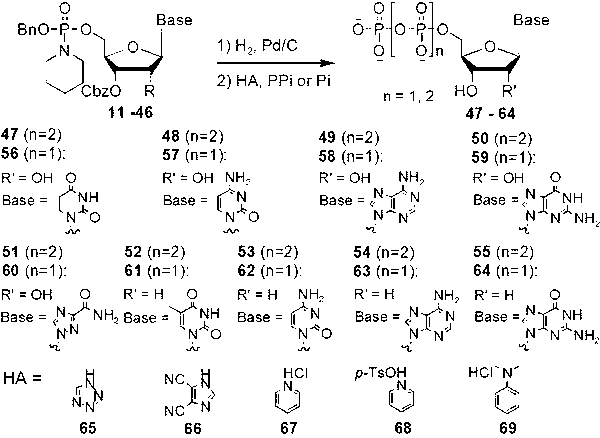Method for synthesizing nucleoside triphosphate and nucleoside diphosphate from all-protected nucleoside phosphoramidite intermediate through acid catalysis
A technology of nucleoside phosphoramide and nucleoside triphosphate, applied in chemical instruments and methods, organic chemistry, production of bulk chemicals, etc., can solve the problem of low synthesis yield, etc., and achieve the effect of improving reaction speed and yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] N -( O -Benzyl- O -(2′,3′-dibenzyloxycarbonyl)uridine-5′-)phosphorylpiperidine (such as figure 1 Medium substance 11 ) synthesis: 2′,3′-dibenzyloxycarbonyluridine (such as figure 1 Medium substance 1 , 500 mg, 0.98 mmol) was dissolved in dry acetonitrile (20 ml), and dry diisopropylethylamine (0.43 ml, 2.5 mmol) was added with stirring. Benzyloxyphosphoramidite diisopropylamine chloride (such as figure 1 Medium substance 10 , 534 mg, 1.95 mmol) was dissolved in anhydrous dry acetonitrile (10 ml), and then slowly added dropwise to the reaction flask. After the dropwise addition was completed, the reaction was continued for 45 minutes. The reaction solution was concentrated under reduced pressure, and the residue was azeotroped twice with acetonitrile (10 ml). A small amount of ethyl acetate was added to the residue to precipitate the resulting diisopropylethylamine hydrochloride. After filtering to remove salt, the filtrate was concentrated to obtain a yellow o...
Embodiment 2
[0020] N -( O -Benzyl- O -(2′,3′-dibenzyloxycarbonyl)adenosine-5′-)phosphorylmorpholine (such as figure 1 Medium substance 31 ) synthesis: 2′,3′-dibenzyloxycarbonyladenosine (such as figure 1 Medium substance 3 , 1.0 g, 1.87 mmol) was dissolved in anhydrous dichloromethane (50 ml), and dry triethylamine (0.65 ml, 4.68 mmol) was added with stirring. Benzyloxyphosphoramidite diisopropylamine chloride (such as figure 1 Medium substance 10, 1.02 g, 3.74 mmol) was dissolved in anhydrous dichloromethane (20 ml), and then slowly added dropwise to the reaction flask. After the dropwise addition was completed, the reaction was continued for 30 minutes. The reaction solution was concentrated under reduced pressure, and the residue was azeotroped twice with 30 ml of acetonitrile. A small amount of ethyl acetate was added to the residue to precipitate the resulting triethylamine hydrochloride. After filtering to remove salt, the filtrate was concentrated to obtain a yellow oil ...
Embodiment 3
[0022] N -( O -Benzyl- O -(2′,3′-dibenzyloxycarbonyl)ribavirin-5′-)phosphoryl-(4-methylpiperidine) (such as figure 1 Medium substance 33 ) synthesis: 2′,3′-dibenzyloxycarbonyl ribavirin (such as figure 1 Medium substance 5 , 1.0 mg, 1.95 mmol) was dissolved in anhydrous dichloromethane (50 ml), and dry tri-n-butylamine (1.16 ml, 4.88 mmol) was added with stirring. Benzyloxyphosphoramidite diisopropylamine chloride (such as figure 1 Medium substance 10 , 1.07 g, 3.90 mmol) was dissolved in anhydrous dichloromethane (20 ml), and then slowly added dropwise to the reaction flask. After the dropwise addition was completed, the reaction was continued for 30 minutes. The reaction solution was concentrated under reduced pressure, and the residue was azeotroped twice with 30 ml of acetonitrile. A small amount of ethyl acetate was added to the residue to precipitate the resulting tri-n-butylamine hydrochloride. After filtering to remove salt, the filtrate was concentrated to o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com