Synthesis method of cinnamate, cinnamonitrile, cinnamamide and derivative thereof
A technology of cinnamic amide and cinnamic acid ester, which is applied in the field of synthesis of cinnamic acid compounds, and achieves the effects of high product yield, simple operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
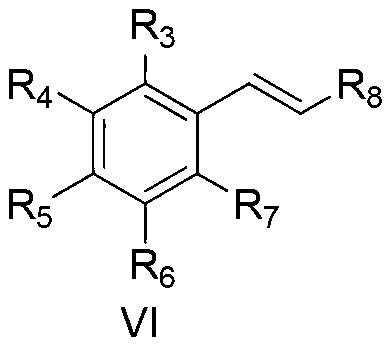
[0021] Taking the preparation of ethyl cinnamate as an example, its structural formula is shown in formula VI, where R 3 , R 4 , R 5 , R 6 , R 7 are hydrogen, R 8 for-COOC 2 h 5 , the raw materials used and the preparation method thereof are as follows:
[0022]
[0023] Add 5.6mg (0.025mmol) palladium acetate and 6.0mg (0.025mmol) 1,2-di-tert-butyl sulfoxide ethane into a 25mL reaction tube, add 1.3g (1mL) dichloromethane, palladium acetate and dichloromethane The mass ratio is 1:232, heated to 45°C, stirred at constant temperature for 5 hours, dichloromethane was distilled off under reduced pressure, and vacuum dried at 35°C for 5 hours to prepare a catalyst.
[0024] Add 53.5mg (0.125mol) tetraphenyltin, 127mg (1mmol) silver fluoride, 100mg (1mmol) ethyl acrylate to 11.6mg (0.025mol) catalyst, catalyst and tetraphenyltin, ethyl acrylate, fluoride The molar ratio of silver is 0.2:1:8:8, inject 2.1g (2mL) of glacial acetic acid with a syringe, the mass ratio of tet...
Embodiment 2
[0026] Taking the preparation of ethyl 4-methoxycinnamate as an example, its structural formula is shown in formula VI, where R 3 , R 4 , R 6 , R 7 are hydrogen, R 5 is methoxy, R 8 for-COOC 2 h 5 , the raw materials used and the preparation method thereof are as follows:
[0027] In Example 1, the tetraphenyltin used is replaced with equimolar 4-methoxytetraphenyltin, and other steps are the same as in Example 1 to prepare ethyl 4-methoxycinnamate, and its yield is 75%, the spectral data of the product is: 1 H NMR (300MHz, CDCl 3 )δ(ppm):7.57(d,J=15.0Hz,1H),7.40(d,J=8.4Hz,2H),6.83(d,J=8.4Hz,2H),6.24(d,J=15.0Hz ,1H),4.18(q,J=7.2Hz,2H),3.76(s,3H),1.26(t,J=7.2Hz,2H); 13 C NMR (75MHz, CDCl 3)δ (ppm): 166.9, 161.0, 143.8, 131.6, 128.6, 126.9, 115.5, 114.059.9, 54.9, 13.9.
Embodiment 3
[0029] Taking the preparation of ethyl 4-fluorocinnamate as an example, its structural formula is shown in formula VI, where R 3 , R 4 , R 6 , R 7 are hydrogen, R 5 is fluorine, R 8 for-COOC 2 h 5 , the raw materials used and the preparation method thereof are as follows:
[0030] In Example 1, the tetraphenyltin used was replaced with equimolar 4-fluorotetraphenyltin, and the other steps were the same as in Example 1 to prepare ethyl 4-fluorocinnamate with a yield of 85%, the product The spectral data of is: 1 H NMR (300MHz, CDCl 3 )δ(ppm):7.56(d,J=15.0Hz,1H),7.45-7.40(m,2H),7.02-6.96(m,2H),6.27(d,J=15.0Hz,1H),4.20( q,J=7.2Hz,2H),1.26(t,J=7.2Hz,3H); 13 C NMR (75MHz, CDCl 3 )δ (ppm): 166.0, 164.6, 161.3, 142.3, 129.8, 129.0, 128.9, 117.2, 117.1, 115.2, 114.9, 59.6, 13.4.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com