Quaternization xylan, preparation method via semi-dry process and application of quaternization xylan
A technology of xylan and quaternization, which is applied to other methods of inserting foreign genetic materials, addition of retention aids, addition of water-repellent agents, etc., can solve the problem of low molecular weight of modified xylan, unfavorable products, and increased production costs and other problems, to achieve the effect of simple preparation process, easy industrialization, and expansion of industrial application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) 1.0g xylan (beech xylan isolated from beech wood, with a molecular weight of 35179mol / g, not purified before use) was dissolved in 1.5ml of 4% NaOH solution at 20°C Alkalization for 40 minutes;
[0028] (2) Add 2 g of 3-chloro-2-hydroxypropyltrimethylammonium chloride to the mixed solution obtained in step (1), mix well, and place it at 90° C. for 3 hours;
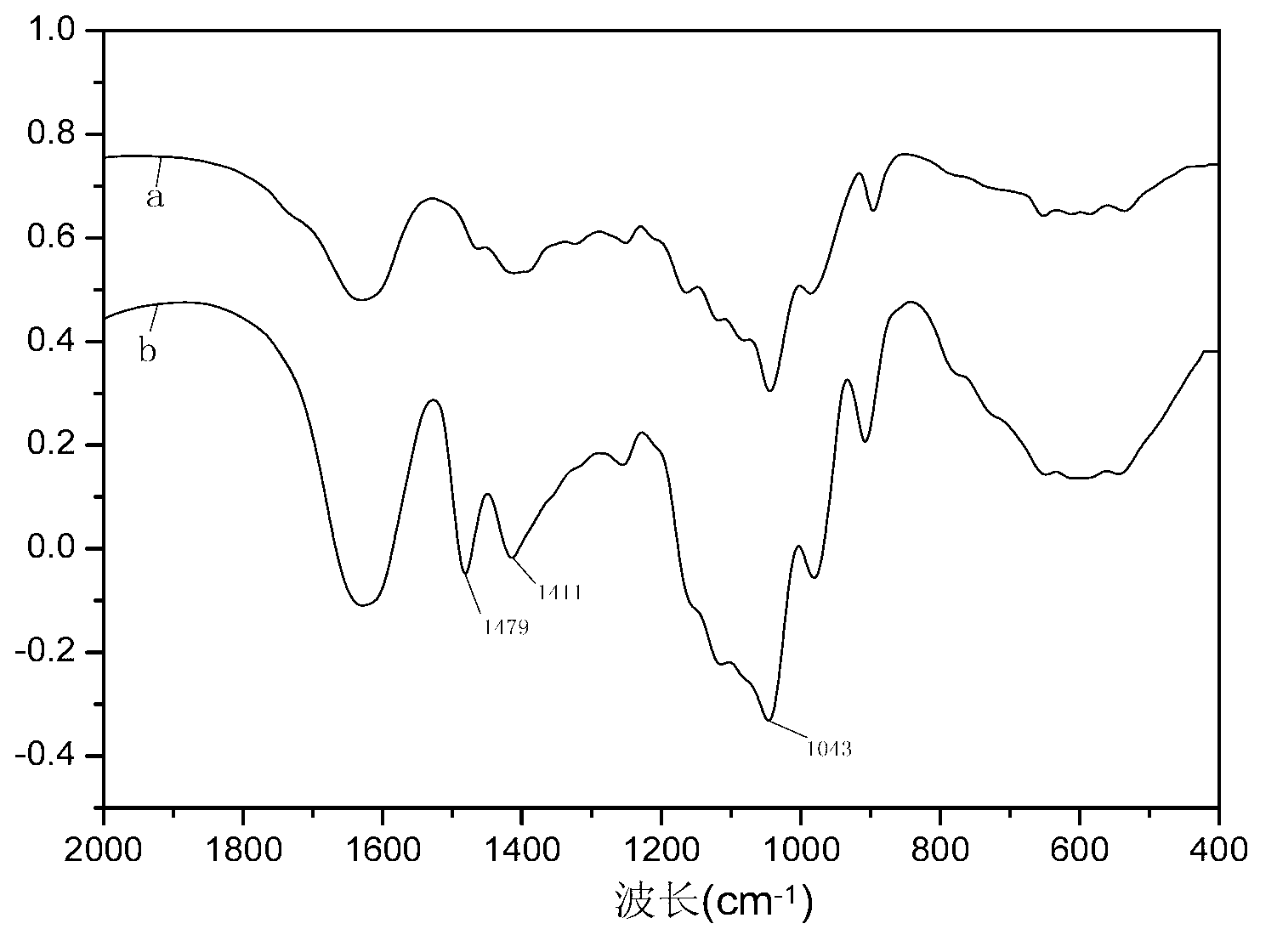
[0029] (3) The product obtained in step (2) was cooled to room temperature, washed once with 50 mL of 70% ethanol, twice with 50 mL of 95% ethanol, and dried at 55° C. for 24 hours to obtain quaternized xylan. figure 1 IR spectrograms of xylan (spectrum a) and quaternized xylan (spectrum b); figure 1 It can be seen that the ether bond is at 1045cm -1 The absorption peak at 1043cm is significantly higher than that of unmodified xylan -1 The absorption peak is large at 1479cm -1 The absorption peak at -CH 2 The signal peak and the methyl group on the substituent. at 1411cm -1 The signal peaks at are generat...
Embodiment 2
[0038] (1) 1.0g xylan (beech xylan isolated from beech wood, with a molecular weight of 38727mol / g, without purification) was dissolved in 1.5ml of NaOH alkali solution with a mass concentration of 10%. 20min;
[0039] (2) Add 4g of 3-chloro-2-hydroxypropyltrimethylammonium chloride to the product obtained in step (1), mix well, and place it at 70°C for 5 hours;
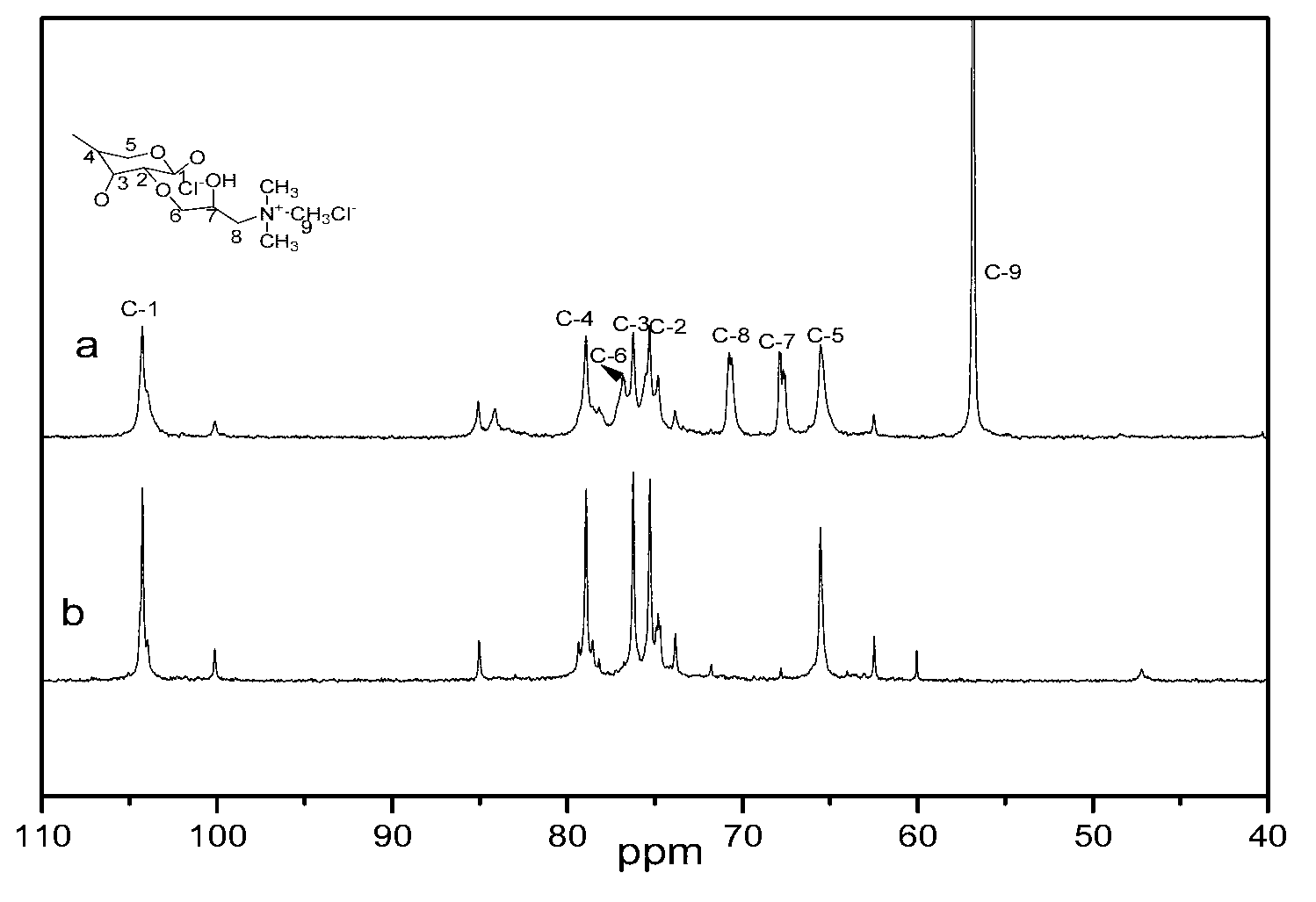
[0040] (3) The product obtained in step (2) was cooled to room temperature, washed once with 50 mL of 70% methanol, twice with 50 mL of 95% ethanol, and dried at 60° C. for 20 h to obtain quaternized xylan. figure 2 NMR spectra of xylan (spectrum b) and quaternized xylan (spectrum a). Compared with xylan (spectrum b), the NMR spectrum a of the modified xylan has a new signal peak, and the strong signal peak at δ56.7 is the C of the quaternary ammonium group. δ (N + -CH 3 ), corresponding to C at δ68.5 β (HOH) and C γ (CH 2 -N + ) signal peak, the weak signal peak at δ73.6 is C α (CH 2 )produced. It can be...
Embodiment 3
[0043] (1) 1.0g xylan (beech xylan isolated from beech wood, molecular weight 32461mol / g, without purification) was dissolved in 1.5ml of NaOH solution with a mass concentration of 24%, and alkalized at 20°C 10min;
[0044](2) Add 8g of 3-chloro-2-hydroxypropyltrimethylammonium chloride to the routine obtained in step (1), mix well, and place it at 50°C for 10h;
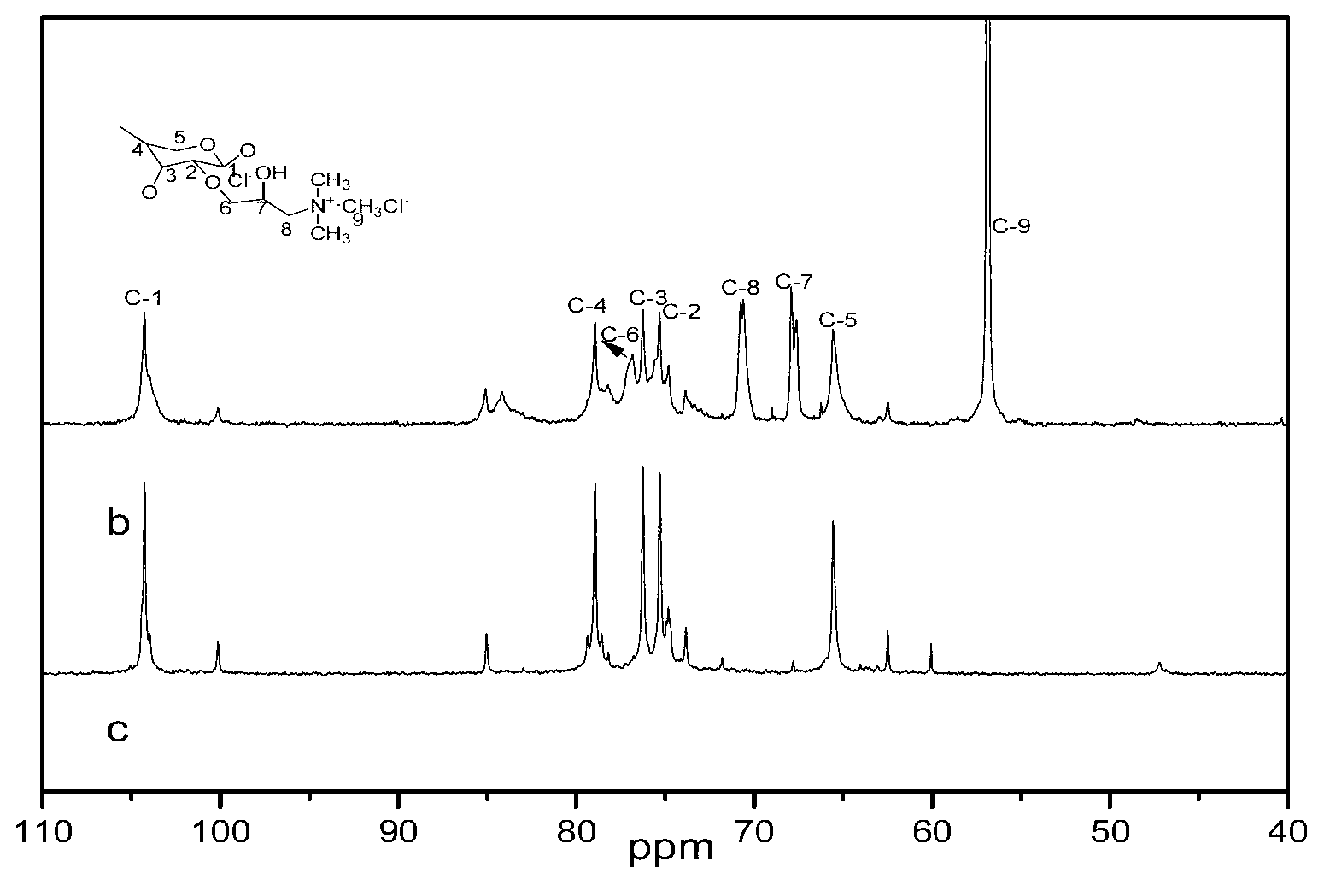
[0045] (3) The product obtained in step (2) was cooled to room temperature, washed once with 50 mL of 70% methanol, twice with 50 mL of 95% ethanol, and dried at 50° C. for 30 h to obtain quaternized xylan. image 3 NMR spectra of xylan (spectrum c) and quaternized xylan (spectrum b). Compared with the xylan nuclear magnetic spectrum (c), the NMR spectrum b of the modified xylan has a new signal peak, and the strong signal peak at δ56.7 is the C of the quaternary ammonium group. δ (N + -CH 3 ), corresponding to C at δ68.5 β (HOH) and C γ (CH 2 -N + ) signal peak, the weak signal peak at δ73.6 is C α (CH 2 )...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com