Alkaline pectinase PelN, as well as encoded gene and application thereof
A technology encoding gene and pectinase, applied in application, genetic engineering, plant genetic improvement, etc., can solve problems such as increasing difficulty and increasing the hardness of plant cell walls.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Embodiment 1, the acquisition of protein PelN and its coding gene
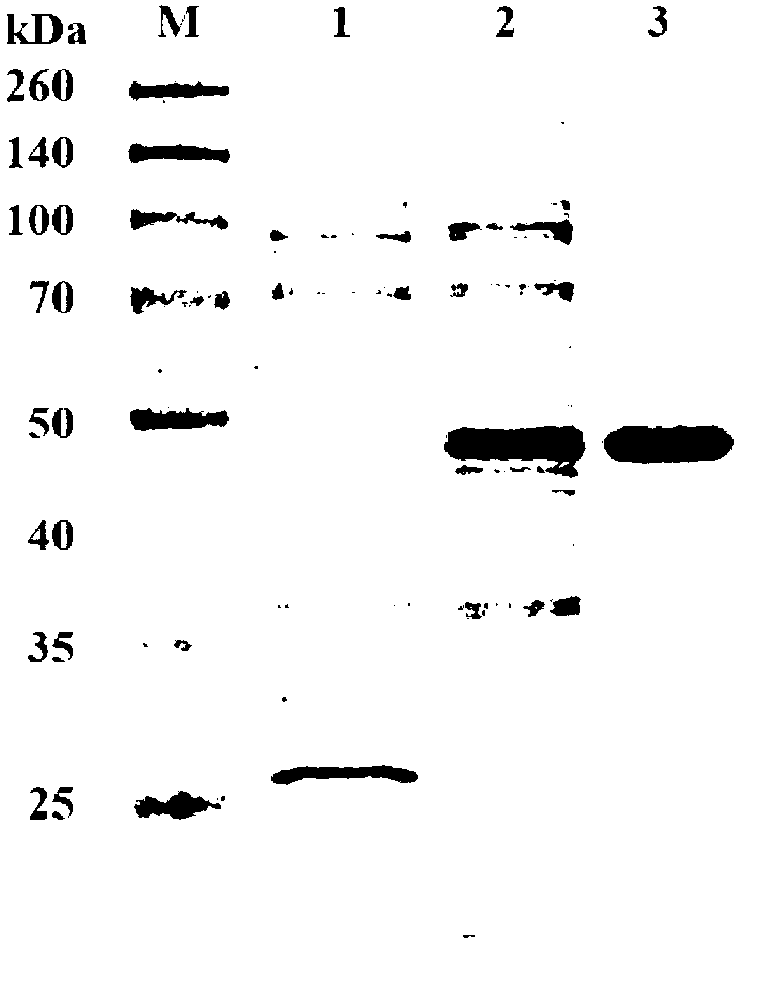
[0051]Using the genomic DNA of Paenibacillus sp. SJN-PL0602 with the preservation number CGMCC NO.5696 in the General Microbiology Center of the China Microbiological Culture Collection Management Committee as a template, PCR amplification was performed with primers F and R, and the amplified The product was subjected to agarose gel electrophoresis, and a 1.4kb fragment was recovered and purified. Sequencing showed that the fragment was 1440bp in size, and its sequence was shown in Sequence Listing Sequence 2, wherein the 7th to 1434th positions of Sequence 2 coded the Sequence Listing sequence The protein shown in 1 is named as PelN, and its coding gene is named as PelN.
[0052] The sequences of the above primers F and R are as follows:
[0053] 5'- CCATGG ATGAAAAAGAACAGTACGAAGCT-3' (the underlined base is the NcoI enzyme recognition sequence);
[0054] 5'- CTCGAG TTAGTAGGAAGTCTTCAGCCAC-3' (the ...
Embodiment 2
[0055] Embodiment 2, the construction of recombinant expression vector and recombinant bacteria
[0056] 1. Digest the DNA fragment of sequence 2 in the sequence listing with NcoI and XhoI double enzymes, and connect it to the backbone fragment of the vector pET22b (purchased from Novagen, catalog number 69744-3) after NcoI and XhoI double enzyme digestion to obtain the recombinant The vector pET22b-PelN was confirmed by sequencing. The recombinant vector pET22b-PelN was inserted between the NcoI and XhoI sites of the vector pET22b by inserting the DNA fragment shown in the 7th to 1434th positions of Sequence 2 of the Sequence Listing.
[0057] 2. Transform the recombinant vector pET22b-PelN obtained in step 1 into Escherichia coli BL21 (DE3) (purchased from Beijing Quanshijin Biotechnology Co., Ltd., the product catalog number is CD601-01), and the obtained recombinant vector pET22b-PelN containing The recombinant bacterium was named BL21(DE3)PelN. The recombinant bacterium w...
Embodiment 3
[0059] Embodiment 3, the preparation of protein PelN
[0060] 1. Preparation of seed solution
[0061] Inoculate the recombinant strain BL21(DE3)PelN obtained in Example 2 into a 250mL Erlenmeyer flask filled with 25mL of seed medium, culture at 37°C, 200r / min, and shake for 6-12h to OD 600nm =6.0, to obtain seed solution;
[0062] The solvent of described seed culture medium is water, and solute and its final concentration in described seed culture medium are respectively: tryptone 12g / L, yeast extract 7g / L, sodium chloride 5g / L; The pH of the base is 7.2.
[0063] 2. Fermentation culture
[0064] Take the seed solution obtained in step 1 and inoculate it into a 250mL Erlenmeyer flask containing 20-30mL of fermentation medium at an inoculum size of 5%; first shake culture at 37°C until OD 600nm =0.6, then add IPTG (inducer, to induce the expression of the target protein PelN) to a final concentration of 200 μM, and continue to shake at 30°C for 40 hours to obtain a fermen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com