High-salinity non-aqueous electrolyte and use thereof
A non-aqueous electrolyte, high salt concentration technology, applied in the direction of circuits, electrical components, secondary batteries, etc., can solve the problems of severe volatilization, poor thermal stability, battery short circuit, etc., and achieve improved battery safety performance, high thermal stability, inhibition dendrite effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] High lithium salt concentration electrolyte system and low lithium salt concentration system are used in the comparison experiment of lithium sulfur battery system:
[0042] High lithium salt concentration electrolyte system:
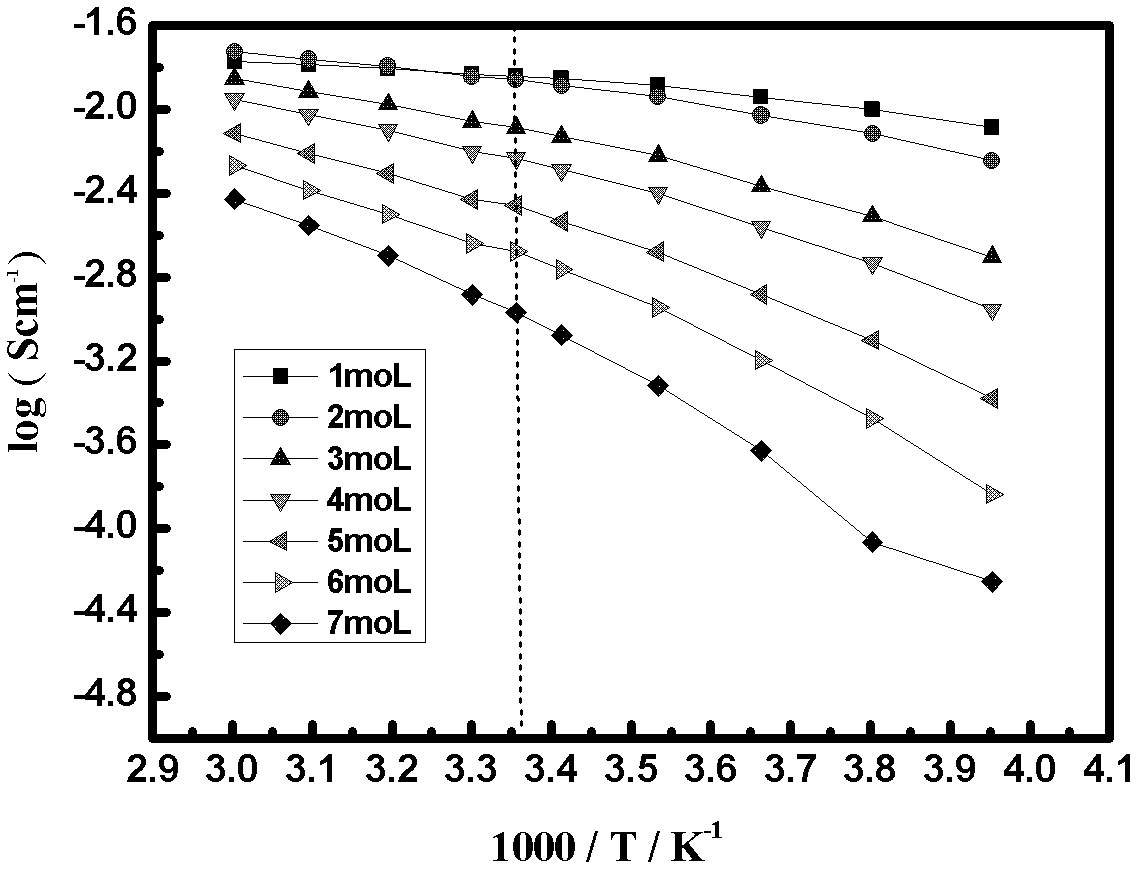
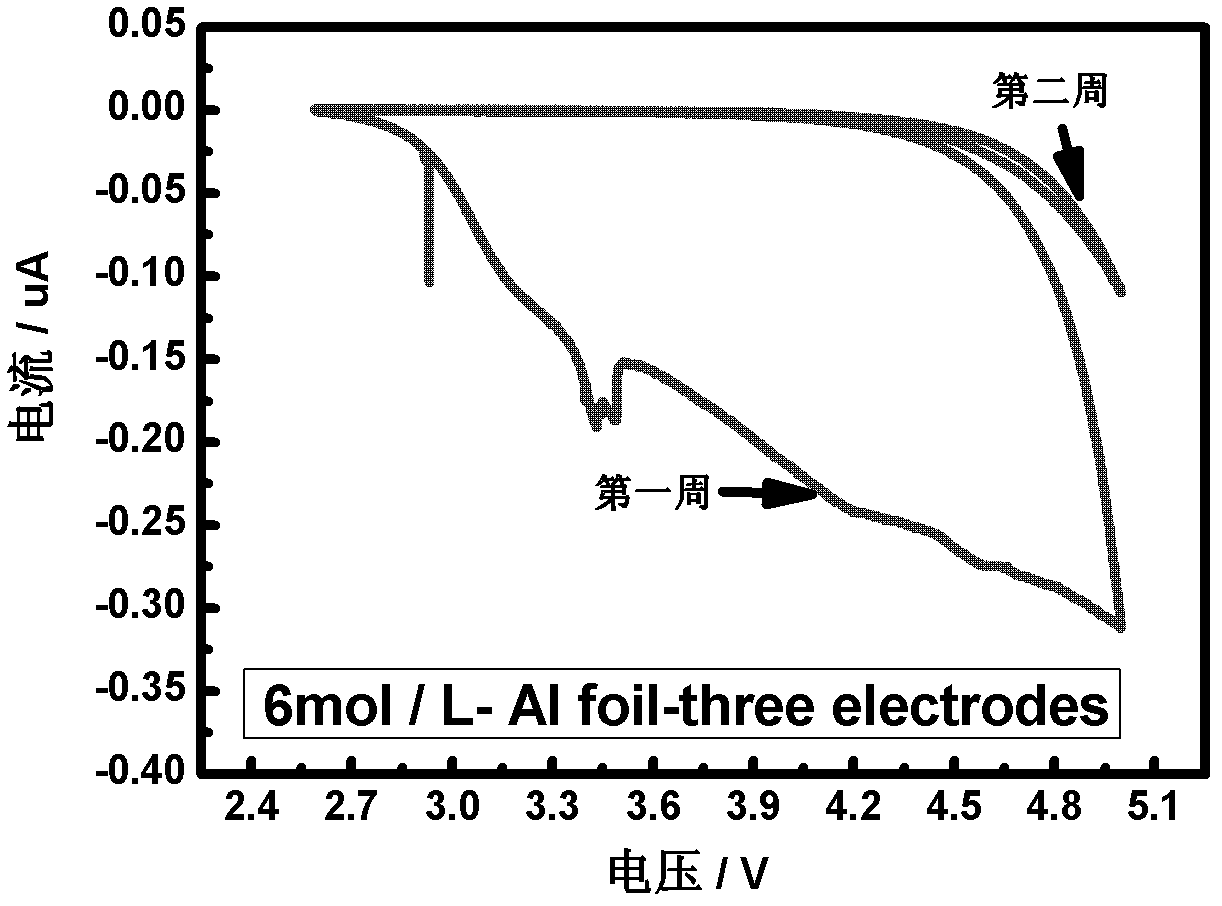
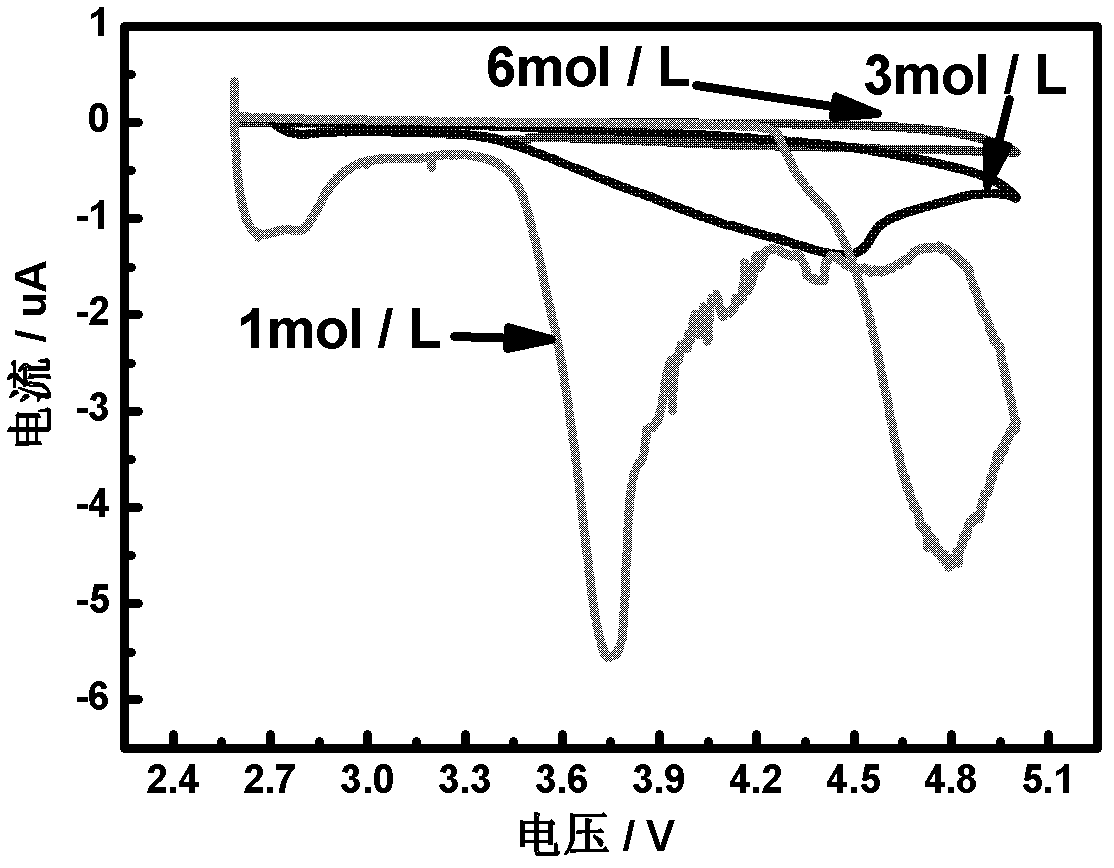
[0043] The electrolytic solution adopts an organic solvent DOL:DME=1:1, the electrolyte is 6mol / L LiTFSI, and the water content of the obtained electrolytic solution is lower than 10ppm.
[0044] Low lithium salt concentration electrolyte system:
[0045] The electrolytic solution adopts an organic solvent DOL:DME=1:1, the electrolyte is 2mol / L LiTFSI, and the water content of the obtained electrolytic solution is lower than 10ppm.
[0046] The lithium-sulfur battery system simulates the battery, and the specific process is as follows:
[0047] Positive electrode material and its pole piece production process:
[0048] The preparation process of the carbon-sulfur composite material is as follows: the porous carbon and the sulfur powder are mix...
Embodiment 2
[0056] High lithium salt concentration electrolyte system and low lithium salt concentration system are used in the comparison experiment of lithium iron sulfide battery system:
[0057] High lithium salt concentration electrolyte system:
[0058] The electrolytic solution adopts an organic solvent DOL:DME=1:1, the electrolyte is 6mol / L LiTFSI, and the water content of the obtained electrolytic solution is lower than 10ppm.
[0059] Low lithium salt concentration electrolyte system:
[0060] The electrolytic solution adopts an organic solvent DOL:DME=1:1, the electrolyte is 1mol / L LiTFSI, and the water content of the obtained electrolytic solution is lower than 10ppm.
[0061] The lithium iron sulfide battery system simulates the battery, and the specific process is as follows:
[0062] Positive electrode material and its pole piece production process:
[0063] Weigh a certain amount of iron sulfide, acetylene black and polyvinylidene fluoride (PVDF) respectively according ...
Embodiment 3
[0067] The high lithium salt concentration electrolyte system is used in the lithium titanate negative electrode system:
[0068] High lithium salt concentration electrolyte system:
[0069] The electrolyte uses organic solvent DEGDME, the electrolyte is 5mol / L LiTFSI, and the water content of the obtained electrolyte is lower than 10ppm.
[0070] Lithium titanate negative electrode battery system simulation battery, the specific process is as follows:
[0071] Positive electrode material and its pole piece production process:
[0072] A certain amount of lithium titanate, acetylene black and polyvinylidene fluoride (PVDF) were respectively weighed according to the weight percentage of 8:1:1, using pyrrolidone as a dispersant, and stirred and mixed evenly. Using aluminum foil as a current collector, the mixed slurry is evenly coated on the current collector, then dried and cut into pole pieces with the same shape and area. The negative pole piece is made of lithium metal. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com