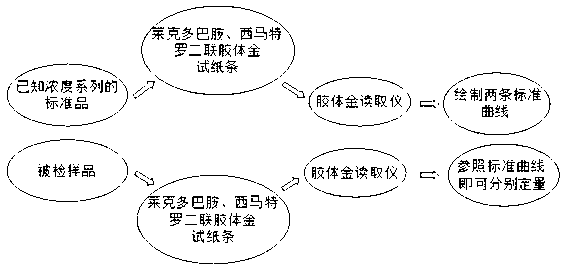
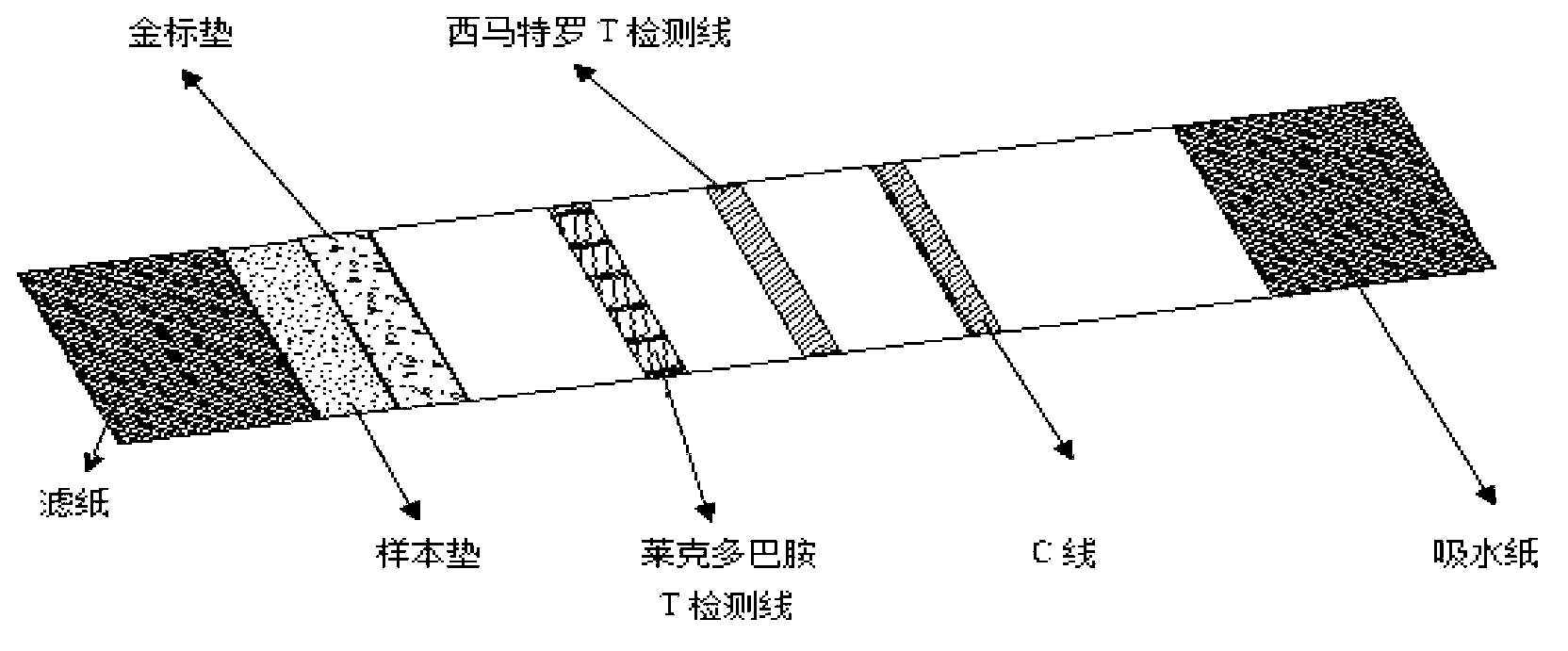
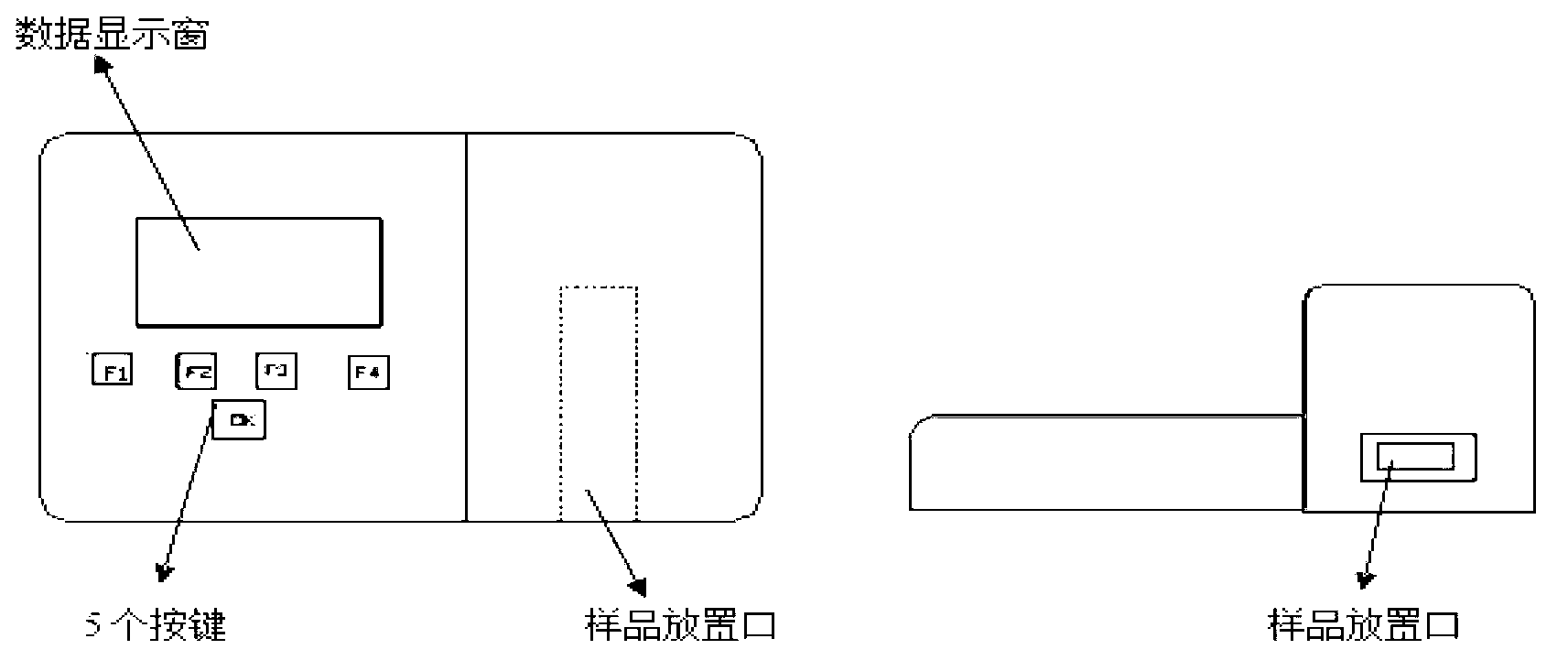
Ractopamine and cimaterolm combined colloidal gold test strip, and preparation method and application thereof
A technology of ractopamine and colloidal gold test paper, which is applied to the detection of β2-receptor agonist residues and the field of food safety, can solve the problems of large background interference, the need for improvement of ractopamine and cimaterol, and the inability to achieve quantitative detection. The effect of uniform size and fixed position
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Synthesis of embodiment 1 immune antigen
[0040] 1.1 Synthesis of ractopamine immune antigen
[0041]Prepare ractopamine-BSA immunoantigen by mixed acid anhydride method: Weigh 34 mg of ractopamine and 10 mg of succinic anhydride to react in 2 mL of pyridine, stir overnight at room temperature, and place pyridine in a fume hood to completely evaporate. The reaction product is ractopamine-BSA Hemisuccinic anhydride; dissolve it in 2 mL of N,N-dimethylformamide and 2 mL of 1,4-dioxane mixture, add 26.2 μL of tri-n-butylamine, stir in an ice bath for 10 minutes, and then Add 15L of isobutyl chloroformate, react at room temperature, stir for 1 hour; add this mixture dropwise to a pre-cooled protein solution (100mg BSA dissolved in 0.1M sodium borate pH8.5), react overnight at room temperature, and dialyze in PBS for 72 hours Above, the purified ractopamine-BSA immune antigen can be obtained after dialysis.
[0042]
[0043] 1.2 Synthesis of cimaterol immune antigen
...
Embodiment 2
[0046] Example 2 Preparation and Titer Detection of Immunogen Monoclonal Antibody
[0047] 2.1 Preparation of ractopamine monoclonal antibody
[0048] Take 8-week-old female BALB / c mice, and use 0.1mL ractopamine-BSA and an equal volume of complete Freund's adjuvant emulsion to immunize each mouse for the first time by intraperitoneal injection; after that, take the same dose of immunogen to add With incomplete Freund's adjuvant, the same method was used to boost immunization once every month; the antibody titer was measured by indirect ELISA 10 to 14 days after the third and fourth immunizations, and finally the one with the highest antibody titer was selected for cell fusion. Spleen cells from immunized mice were taken under sterile conditions, fused with SP2 / O myeloma cells at a ratio of 5:1, added to HAT medium, 37 degrees Celsius, 6% CO 2 Cultivate in an incubator; replace half of the medium with fresh HAT medium after 5 days, and replace the HAT medium with HT medium af...
Embodiment 3
[0057] Embodiment 3 Preparation of ractopamine and cimaterol double colloidal gold test strips in the present invention
[0058] 3.1 Preparation of colloidal gold
[0059] The basic principle of preparing immune colloidal gold is that chloroauric acid can be polymerized into gold particles of a certain size under the action of a reducing agent to form a negatively charged hydrophobic colloid solution that is stable due to electrostatic interaction. The present invention adopts trisodium citrate reduction method to prepare colloidal gold, and specific process is as follows: take 0.01% chloroauric acid 100mL aqueous solution and heat to boiling, accurately add 1.5mL of 1% trisodium citrate aqueous solution under agitation, golden chloroauric acid is in Turn into purple within 2 minutes, turn off the heat source, continue to stir at high speed for 10 minutes, then reduce the speed to low gear, continue to stir for 1 hour, and restore the original volume with distilled water after...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com