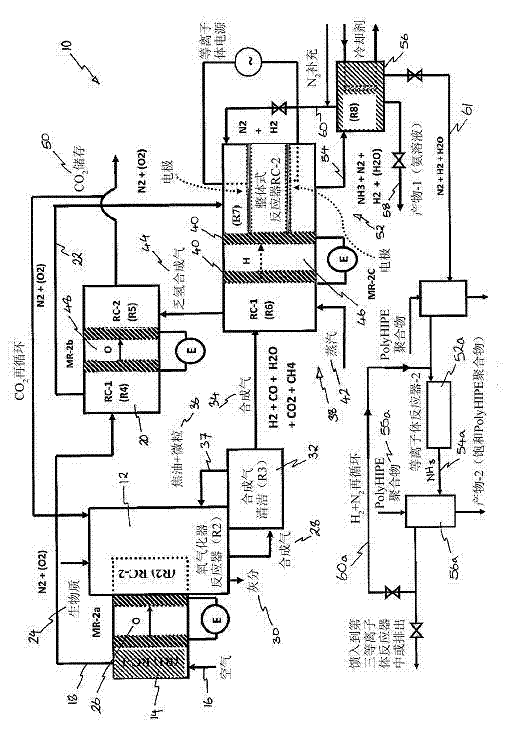
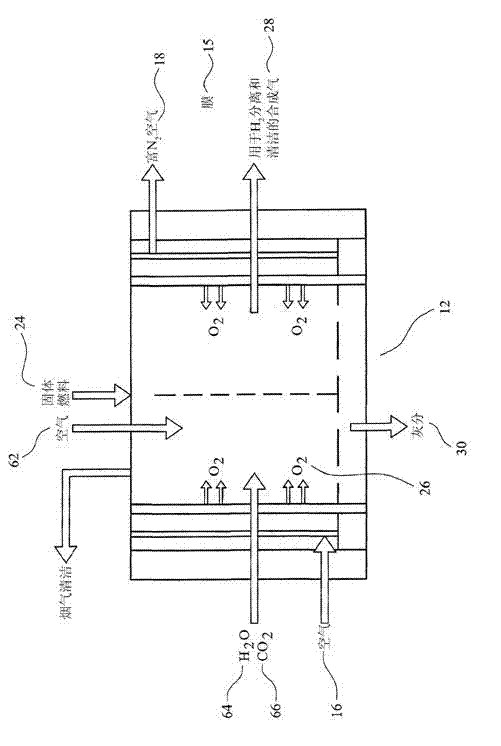
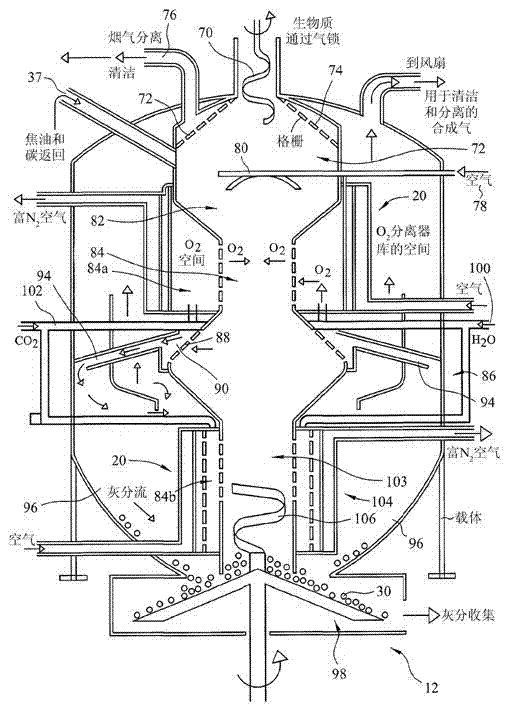
Ammonia production by integrated intensified processes
A production method and synthesis gas technology, applied in the field of ammonia production, can solve low efficiency and other problems, and achieve the effect of improving ammonia production efficiency, stabilizing machinery and thermal adsorption machinery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0133] In the catalytic plasma synthesis of ammonia and its recovery, the following steps are taken: a) Preparation of solid acid and water absorbent, which is ultimately used as a synthetic rhizosphere in the soil to promote plant growth; b) Preparation is suitable for plasma reaction The grading of the pores in the reactor and the structured catalyst with high surface area; c) The high dielectric constant catalyst is used in the plasma reactor, where at least one electrode is in contact with the catalyst; d) The plasma reactor is configured to make the ammonia yield Maximize and minimize energy consumption. The following examples are provided to illustrate these steps.
example A
[0134] Example A. Preparation of PolyHIPE polymer for ammonia and water adsorption and formation of synthetic rhizosphere (SRS) media and slow release of fertilizer materials for the preparation of synthetic rhizosphere (SRS) media polymer
[0135] All chemicals are reagent grade. Monomer (styrene), crosslinking agent (divinylbenzene, DVB), polymerization initiator (potassium persulfate), sulfonating agent (0.97 g sulfuric acid / g solution) are all supplied by Aldrich.
[0136] Preparation of synthetic rhizosphere media polymer
[0137] SRS media polymer is a nanostructured cross-linked hydrophilic elastic ionic microporous material, generally called PolyHIPE polymer (PHP), which is prepared by the high internal phase emulsion (HIPE) polymerization and subsequent functionalization route as disclosed by Akay et al. in US Patent 7 820 729. The preparation of functionalized PHP has 3 stages: 1) stable HIPE formation; 2) polymerization; and 3) functionalization.
[0138] Preparation of...
example B
[0147] Example B. Preparation of nanostructured microporous supported catalyst
[0148] The prior art related to the preparation of supported metal catalysts uses particulate porous supports such as aluminum oxide (Al 2 O 3 ; Alumina) or silicon oxide (SiO 2 ; Silica). An aqueous solution of metal catalyst precursor salts (such as nickel, cobalt, iron, ruthenium nitrates) is used to impregnate the support, and the resulting system is heat-treated at high temperature (typically 600°C) to decompose the catalyst precursor salt to obtain metal oxides , And then reduce it at a similar temperature to reduce the metal oxide to an active metal catalyst.
[0149] We have found that instead of using porous solid catalyst supports, high surface area supported catalysts can be obtained by using stable nano-scale support particles dispersed in water, and the dispersion also contains catalyst precursor salts such as Ni(NO 3 ) 2 ), this is because the nitrate of the catalytic metal is highly sol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Surface area | aaaaa | aaaaa |
| Surface area | aaaaa | aaaaa |
| Surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com