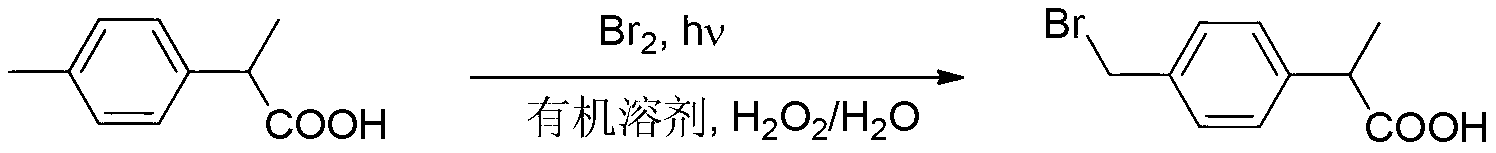Environment-friendly preparation method of 2 - (4 - Bromomethylphenyl) propionic acid based on two-phase free radical reaction
A bromomethylphenyl, green and environmentally friendly technology, applied in the field of preparation of pharmaceutical intermediates, can solve problems such as pollution, achieve the effects of reducing reaction volume, reducing synthesis costs, and simplifying production processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
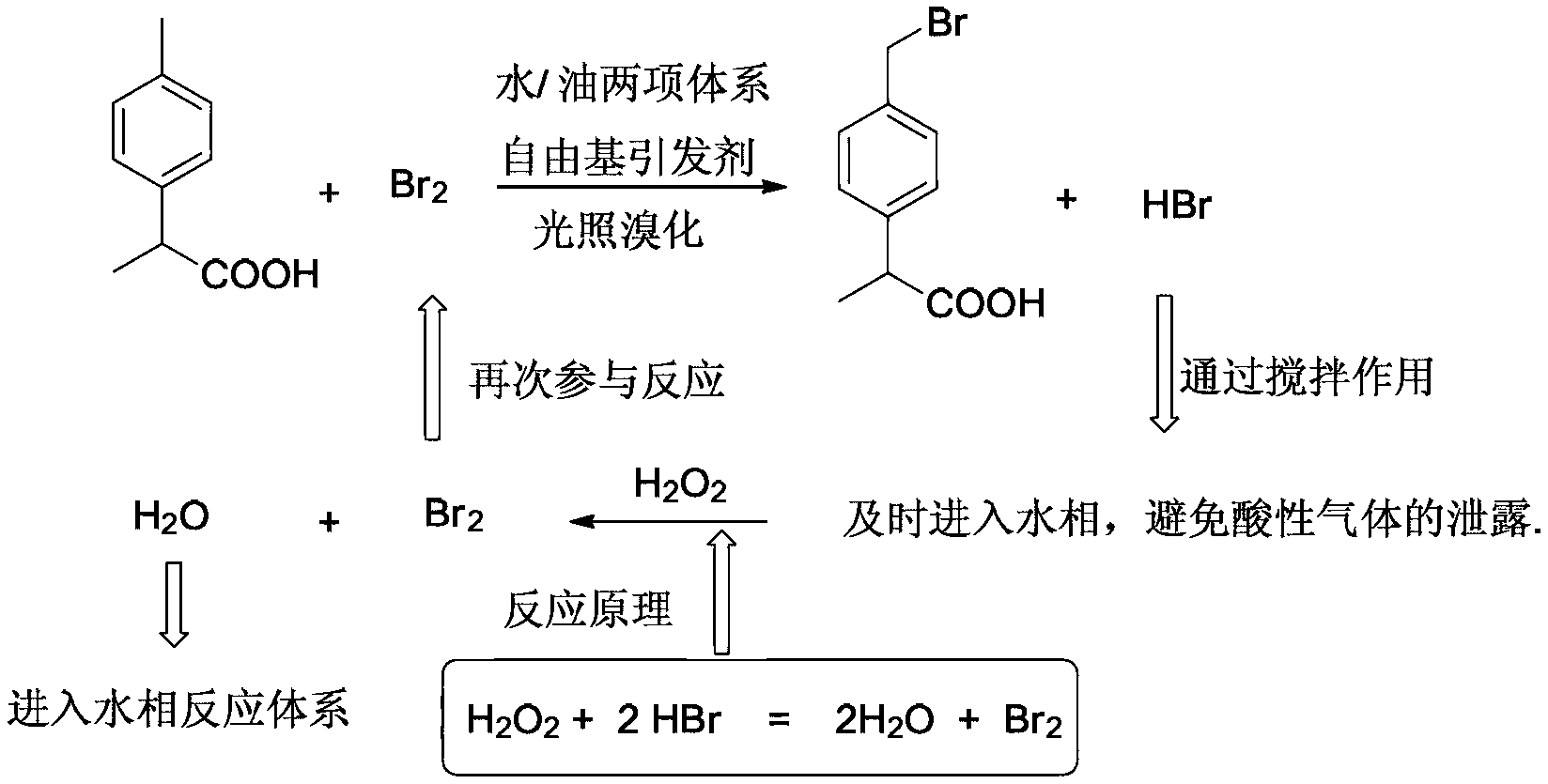
[0057] Embodiment 1 adopts the green synthesis of the two-phase reaction system of free radical initiator in combination with illumination method as follows:
[0058] Prepare bromine solution in advance as follows: add benzoyl peroxide (BPO, 0.82 g, 3.39 mmol, as a free radical initiator), dichloromethane (45.0 g, 36.0 mL), bromine (9.50g, 3.05mL, 0.059mol), shake to mix well.
[0059] Take 2-(4-methylphenyl)propionic acid (16.4g, 0.10mol) and put it into a three-necked flask equipped with a temperature agent, a reflux condenser and a stirrer, and add dichloromethane (125.3g, 97.4mL ), water (32.8g, 32.8mL), stirring and lowering the temperature to 10-20°C, and then turning on an 80W energy-saving lamp to irradiate the reaction solution;
[0060] Under the condition of 10-20°C, slowly add the pre-prepared bromine solution dropwise, and the rate of addition should be such that the color of the reaction solution is light yellow to reddish. After more than half of the amount of...
Embodiment 2
[0062] Embodiment 2 adopts dichloroethane to replace dichloromethane, and carries out the synthesis of light method combined with free radical initiator
[0063] The basic operation process, the amount of raw materials involved, the light-induced reaction and the post-treatment method are the same as in Example 1, except that the two parts are used as organic solvents for the organic phase. Dichloroethane is used instead of dichloromethane, dichloroethane The amount of dichloroethane used to dissolve 2-(4-methylphenyl)propionic acid is (130.0g, 100.0mL), and the amount of dichloroethane used to dilute bromine is (43.5g, 32.6mL). mp=128.0~130.5℃, the yield is 82.0%, the purity is 98.8%, and the spectral identification data is the same as above.
Embodiment 3
[0064] Embodiment 3 adopts the green synthesis of the two-phase reaction system of direct heating method in conjunction with free radical initiator as follows:
[0065] Pre-constitute the bromine solution as follows: add benzoyl peroxide (BPO, 0.82 g, 3.39 mmol, as a free radical initiator), dichloroethane (43.5 g, 32.6 mL), bromine element (9.50g, 3.05mL, 0.059mol), shake to mix well.
[0066] Get 2-(4-methylphenyl) propionic acid (16.4g, 0.10mol) and put into the three-neck flask that temperature agent, reflux condenser and stirrer are housed, add dichloroethane (130.0g, 100.0 mL), water (32.8g, 32.8mL), appropriate amount of hydrogen peroxide, fully stirred, and after the product was completely dissolved, heated to reflux; at the same time, inject the pre-prepared bromine solution into the constant pressure dropping funnel, and reflux, Slowly add while adding, the rate of addition is preferably when the color of the reaction solution is light yellow to reddish;
[0067] A...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com