Preparation method of anode catalyst for sodium borohydride fuel battery
A fuel cell, sodium borohydride technology, applied in physical/chemical process catalysts, metal/metal oxide/metal hydroxide catalysts, battery electrodes, etc., can solve the problem of hindering mass transfer, intensifying anode polarization, and reducing fuel utilization rate and other issues, to achieve the effect of improving utilization, high cost performance, and improving security and reliability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
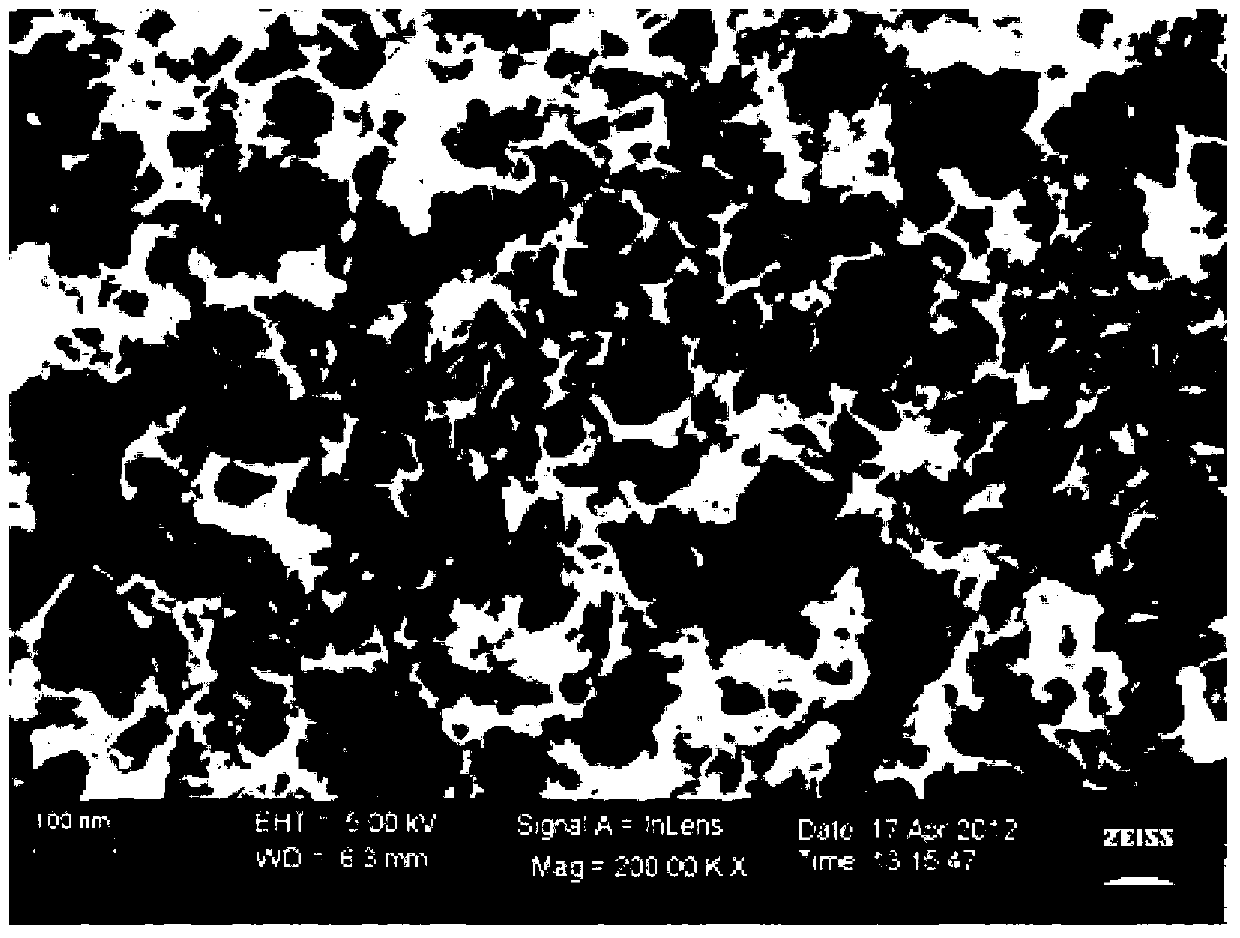
[0025] Example 1: Preparation of macroporous carbon
[0026] Weigh the hydrophilic nano-CaCO according to the mass ratio of 1:1 3 (particle size 15-40 nm) and 10 g of glucose each, add 100 mL of deionized water, and mix with ultrasonic vibration for 30 minutes to dissolve the glucose and mix with nano-CaCO 3 Disperse evenly, heat to evaporate water, and then cure at 160°C for 6 hours. The cured product was heated to 800 °C under the protection of nitrogen atmosphere, and carbonized at constant temperature for 2 hours. The carbonized product was washed successively with hydrochloric acid and deionized water, and dried at a constant temperature of 120°C for 4 hours to obtain a macroporous carbon material, whose morphology was as follows: figure 1 shown.
Embodiment 2
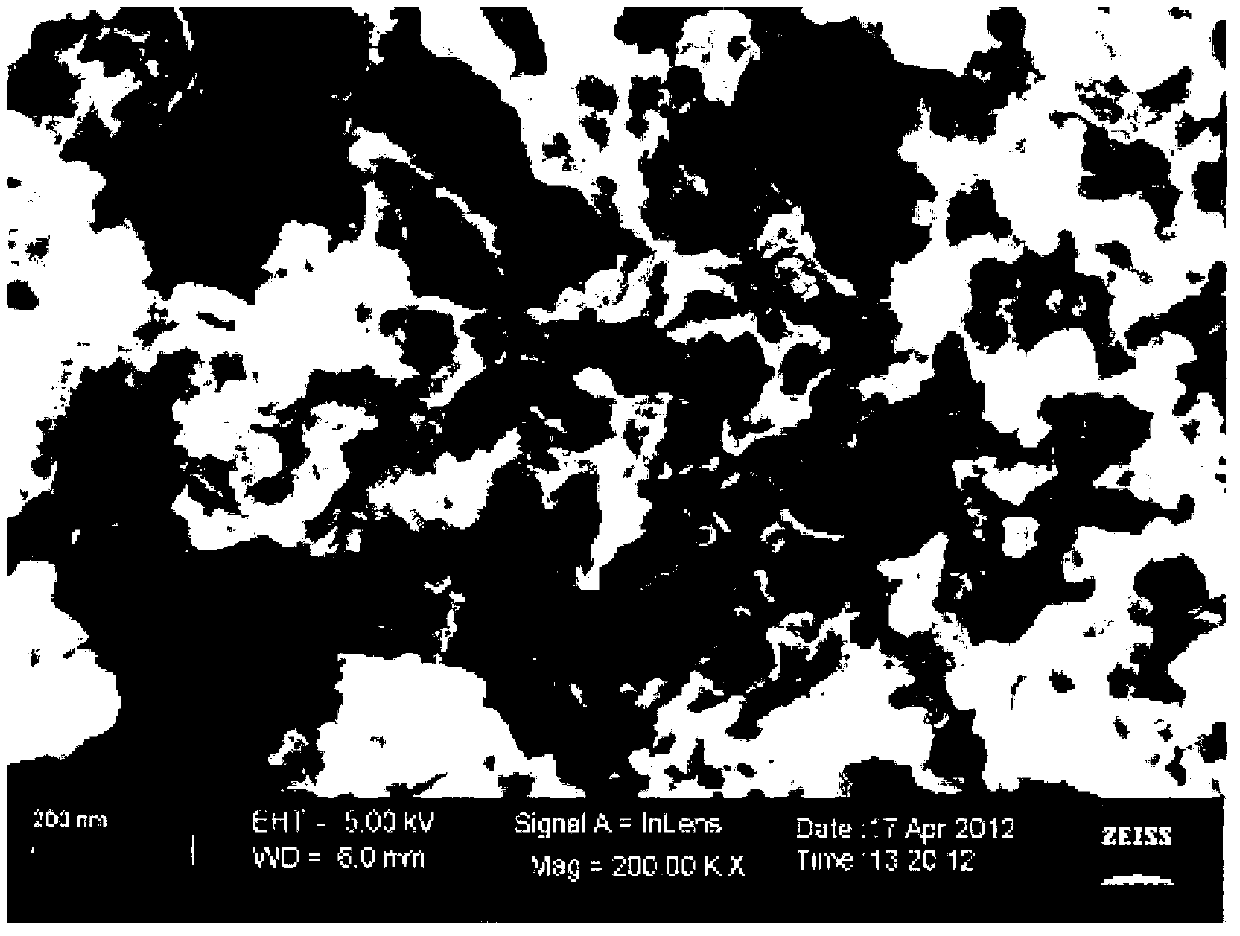
[0027] Example 2: Preparation of polypyrrole modified macroporous carbon
[0028] Weigh the hydrophilic nano-CaCO according to the mass ratio of 1:1 3 (15-40 nm) and 10 g of sucrose each, add 100 mL of deionized water, and mix with ultrasonic vibration for 30 minutes to dissolve the glucose and mix with nano-CaCO 3 Disperse evenly, heat to evaporate water, and then cure at 160°C for 6 hours. The cured product was heated to 800 °C under the protection of nitrogen atmosphere, and carbonized at constant temperature for 2 hours. The carbonized product was washed successively with hydrochloric acid and deionized water, and dried at a constant temperature at 120° C. for 4 hours to obtain a macroporous carbon material.
[0029]The macroporous carbon material was crushed to a particle size of 100-400 mesh, and 1 g of macroporous carbon was put into a three-neck flask, vacuumed for 2 hours, and 60 mL of pyrrole solution was added with a separatory funnel, which contained 16.77 g of ...
Embodiment 3
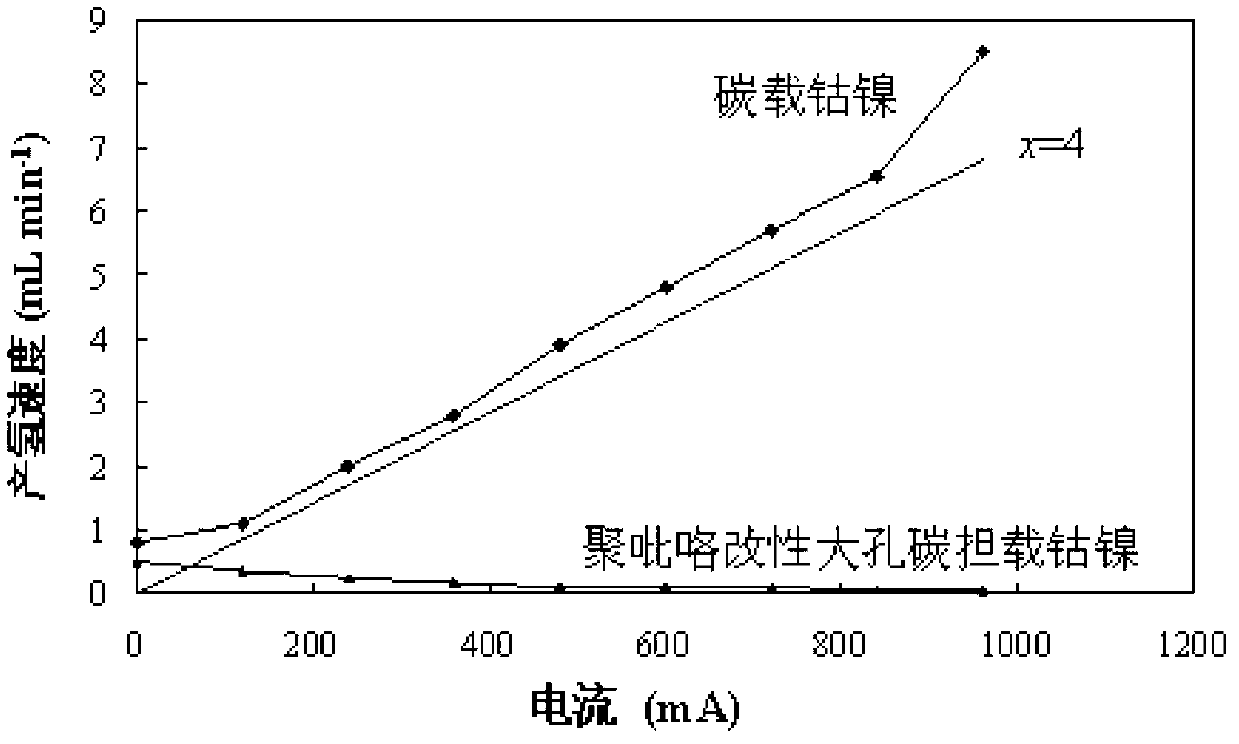
[0030] Example 3: Polypyrrole-modified macroporous carbon-supported platinum catalyst
[0031] 1 g of polypyrrole-modified macroporous carbon material prepared in Example 2 was placed in a hydrothermal reactor with a volume of 150 mL, and 100 mL of platinum chloride solution was added, containing 1.2 mmol of platinum chloride (0.32 g) After ultrasonic vibration and mixing for 20 minutes, seal the reaction vessel, place it in an oil bath, raise the temperature of the oil bath to 300 °C for 12 hours, filter, wash with deionized water, and vacuum dry at 90 °C to obtain macroporous carbon loading Nitrogen-containing platinum catalyst.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com