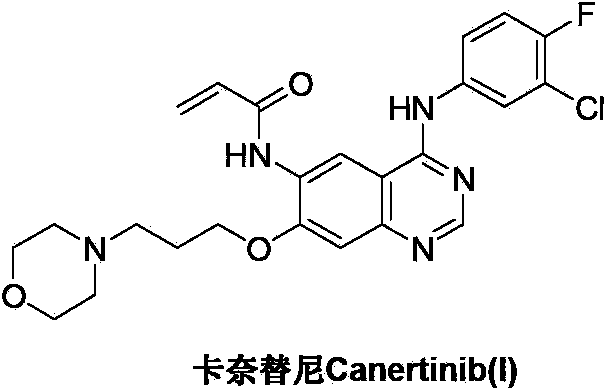
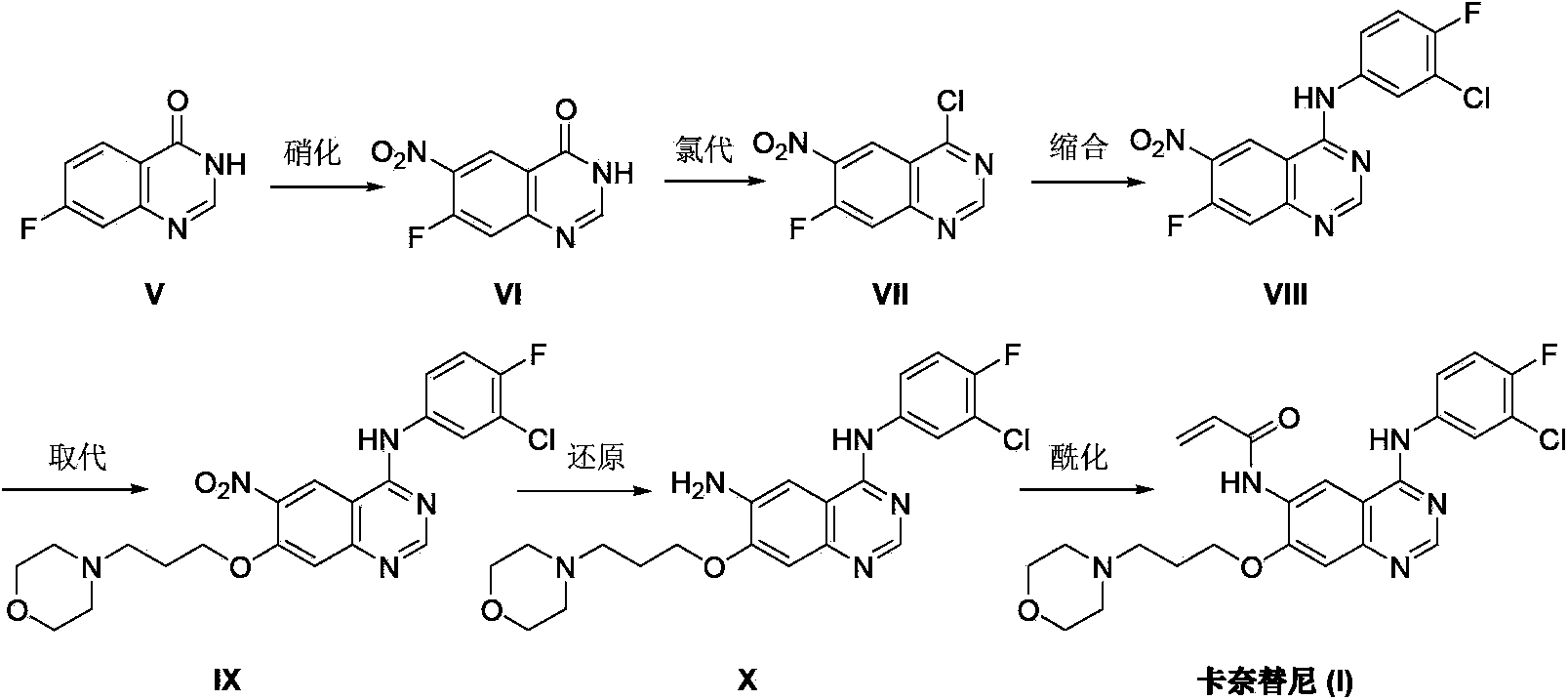
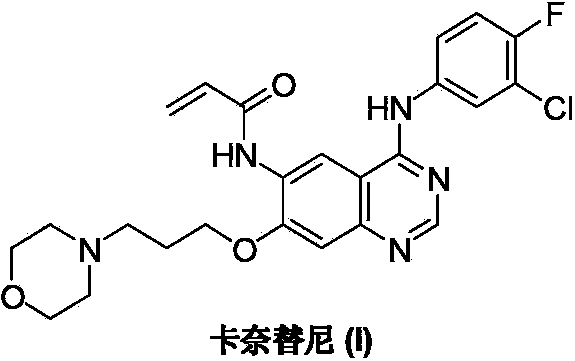
Preparation method of canertinib (I)
A canetinib and morpholine-based technology, applied in the field of preparation of canetinib, can solve the problems of many steps, unsuitable requirements for industrialization, and difficulty in obtaining raw materials, and achieves easy availability of raw materials, promotion of development, and promotion of economy. effect of technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] At room temperature, add diisopropyl azodicarboxylate (3mL, 15mmol) and 5mL of tetrahydrofuran into a 100mL three-necked flask, add dropwise a solution of triphenylphosphine (4.0g, 15mmol) in 25mL of tetrahydrofuran at room temperature, and keep at room temperature for reaction 2 Hour. Under nitrogen protection, 3-(4-morpholinyl)-1-propanol (0.5g, 3.4mmol) in tetrahydrofuran 5mL solution was added dropwise to the above reaction system, after the addition was complete, 4-chloro- 6-Amino-7-hydroxyquinazoline (II) (0.59 g, 3.0 mmol), stirred at room temperature for 4 hours. A 5 mL solution of 3-(4-morpholino)-1-propanol (0.38 g, 2.6 mmol) in tetrahydrofuran was added dropwise, and the reaction was continued at room temperature for 2 hours, and the reaction was completed by TLC monitoring. The solvent was recovered by distillation under reduced pressure, the residue was adjusted to pH=5-6 with dilute hydrochloric acid, extracted with ethyl acetate, and the organic phase wa...
Embodiment 2
[0029] Add 4-chloro-6-amino-7-[3-(4-morpholinyl)propoxy]quinazoline (III) (0.81g, 2.5mmol), triethylamine (0.25g , 2.5mmol) and 20mL of dichloromethane, heated to 40-45°C, stirred until the system was dissolved uniformly. When the temperature was lowered to below 10°C, a solution of acryloyl chloride (0.20 g, 2.8 mmol) in 10 mL of dichloromethane was slowly added dropwise. After the drop was completed, the reaction was continued at room temperature for 6 hours, and the reaction was detected by TLC. The reaction solution was washed with 10% sodium bicarbonate solution and water, and dried over anhydrous sodium sulfate. The solvent was recovered under reduced pressure, and the residue was recrystallized from ethyl acetate to obtain 4-chloro-7-[3-(4-morpholino)propoxy]-6-acrylamidoquinazoline (IV) as a pale yellow solid 0.80g, yield 85.1%.
Embodiment 3
[0031] Add 4-chloro-7-[3-(4-morpholinyl) propoxy]-6-acrylamidoquinazoline (IV) (1.13g, 3.0mmol), triethylamine ( 0.45g, 4.5mmol) and isopropanol 30mL, stirred until the system was dissolved uniformly. A solution of 4-fluoro-3-chloroaniline (0.48 g, 3.3 mmol) in 20 mL of isopropanol was slowly added dropwise. After the drop was completed, the reaction was continued at room temperature for 12 hours, and the reaction was detected by TLC. The reaction solution was poured into 100 mL of ice water and filtered. After the filter cake was dried, it was recrystallized with dimethyl sulfoxide to obtain 1.25 g of canertinib (I) as a pale yellow solid with a yield of 85.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com