Second-order nonlinear optical polyarylester material, and synthetic method and application thereof
A second-order nonlinear, polyarylate technology, applied in optics, optical components, instruments, etc., can solve the problems of poor solubility, fast decay of electro-optical activity, difficult thin-film devices, etc. easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
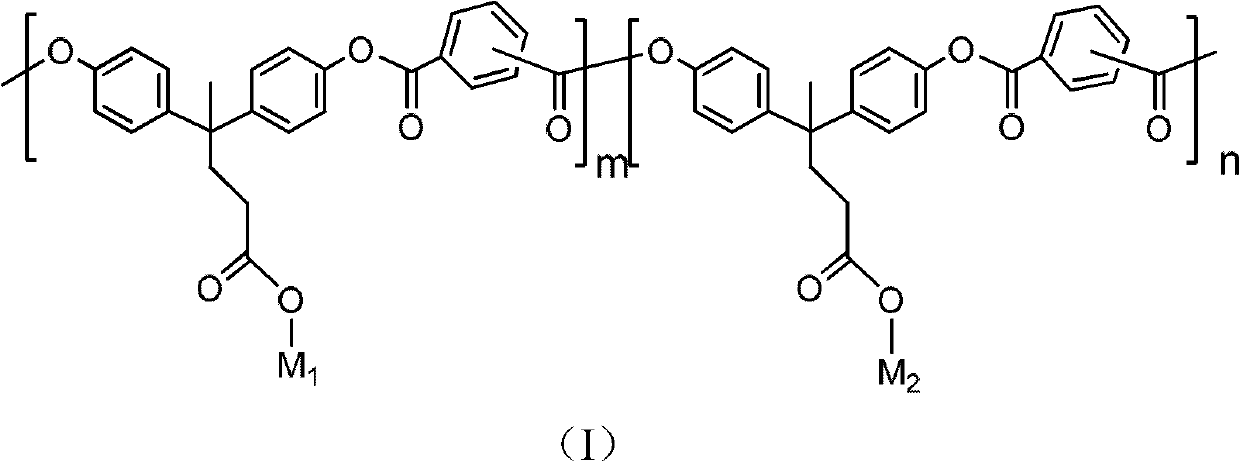
[0080] Synthesis of Carboxylated Polyarylate (sPAR)
[0081]
[0082] Take bisphenolic acid (4.72g, 20mmol), dissolve 0.1g of tetrabutylammonium bromide in 100mL sodium hydroxide aqueous solution (containing 60mmol sodium hydroxide), add isophthaloyl dichloride dichloromethane solution under vigorous stirring Medium (20mmol isophthaloyl chloride dissolved in 50mL dichloromethane). React at room temperature for 1 hour, and then adjust the pH of the system to 3 with 2N hydrochloric acid. Pour the solution into a large amount of acetone to precipitate, filter it with suction, wash it repeatedly with deionized water and acetone, and then dry it in vacuum at 50°C for 24 hours. The white polymer obtained is the target product sPAR.
Embodiment 2
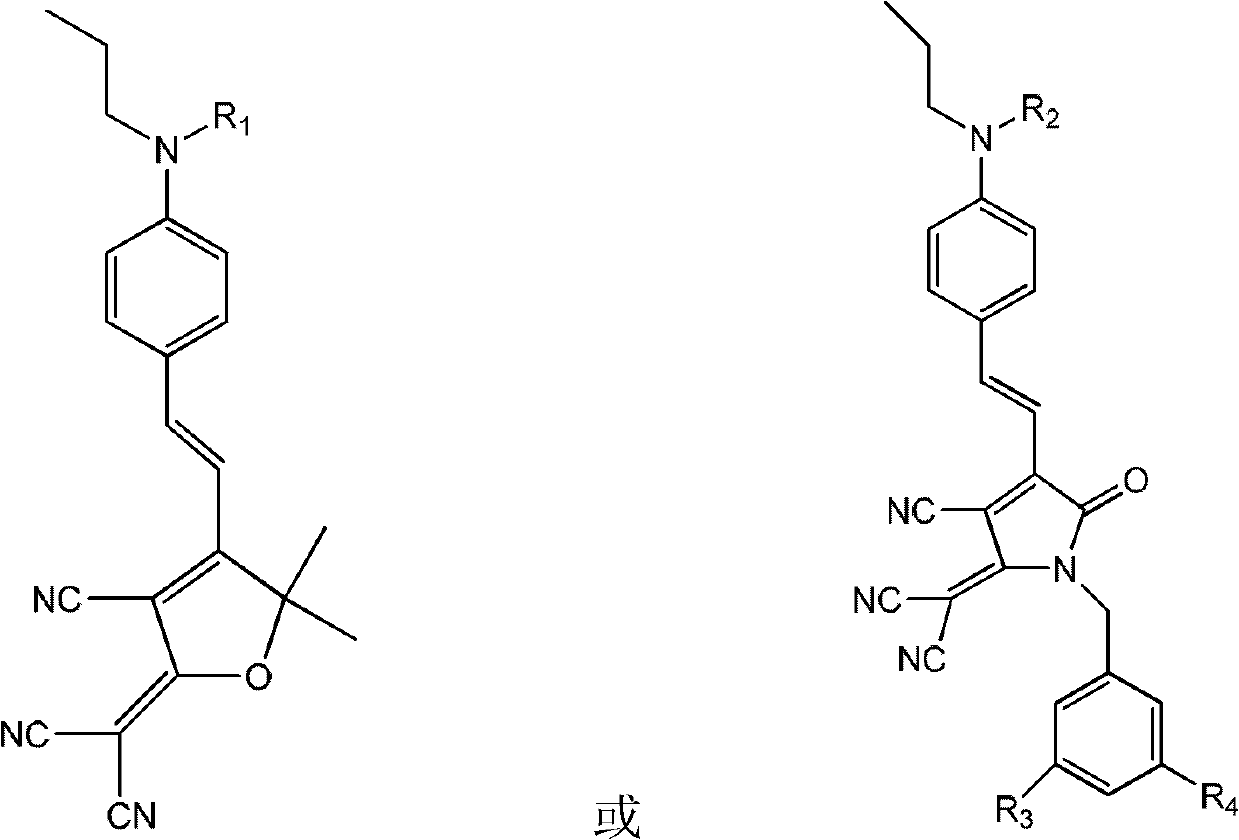
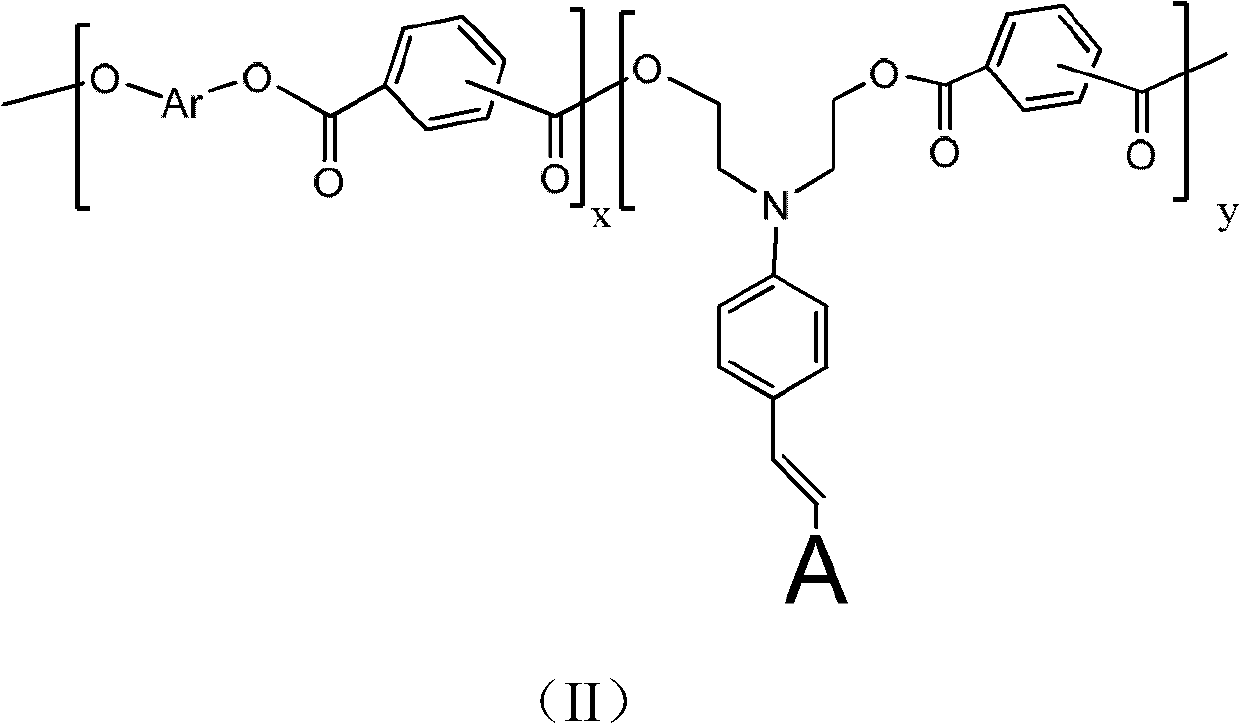
[0084] Synthesis of side-chain second-order nonlinear optical polyarylate sPAR-TCP-1 (M 1 For the chromophore TCP-1, M 2 is anthracenbenzyl, R 3 , R 4 independently alkoxy)
[0085]
[0086] The polyarylate sPAR (2g, 5mmol) of embodiment 1, chromogen TCP-1 (0.69g, 1mmol) are dissolved in the mixed solution of 30mL tetrahydrofuran and 20mL methylene chloride, then add 0.20g N under nitrogen protection , N'-dicyclohexylcarbodiimide and 0.30 g of dimethylaminopyridine p-toluenesulfonate. After reacting at room temperature for 24 hours, 9-anthracenemethanol (0.83 g, 4 mmol) and 0.8 g of N,N'-dicyclohexylcarbodiimide were added. The reaction was continued for 24 hours, and the mixed solution was poured into a large amount of methanol solution to settle. Suction filtration, the solid was dissolved with tetrahydrofuran, and then settled with methanol, and this was repeated several times until the filtrate was nearly colorless. Vacuum drying at 50° C. for 24 hours yielded a d...
Embodiment 3
[0088] Carboxyl-containing polyarylate sPAR (2g, 5mmol) with the structure of Example 1, and the chromophore TCP-1 (0.04g, 0.05mmol) with the structure of Example 2 were dissolved in the mixed solution of 30mL THF and 20mL methylene chloride , and then added 0.01 g of N, N'-dicyclohexylcarbodiimide and 0.02 g of dimethylaminopyridine p-benzenemethanesulfonate under nitrogen protection. After reacting at room temperature for 24 hours, 9-anthracenemethanol (1.03 g, 4.95 mmol) and 1 g of N,N'-dicyclohexylcarbodiimide were added. The reaction was continued for 24 hours, and the mixed solution was poured into a large amount of methanol solution to settle. Suction filtration, the solid was dissolved with tetrahydrofuran, and then settled with methanol, and this was repeated several times until the filtrate was nearly colorless. Vacuum drying at 50°C for 24 hours gave a light green solid which was the target product sPAR-TCP1. UV-Vis (tetrahydrofuran): λ max =702nm; IR (thin film)...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com