Preparation method of lithium titanate
A technology of lithium titanate and lithium titanate, applied in electrolysis process, electrolysis components, etc., can solve problems such as low utilization rate of lithium titanate, solve product batch stability and consistency problems, and controllable particle size Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
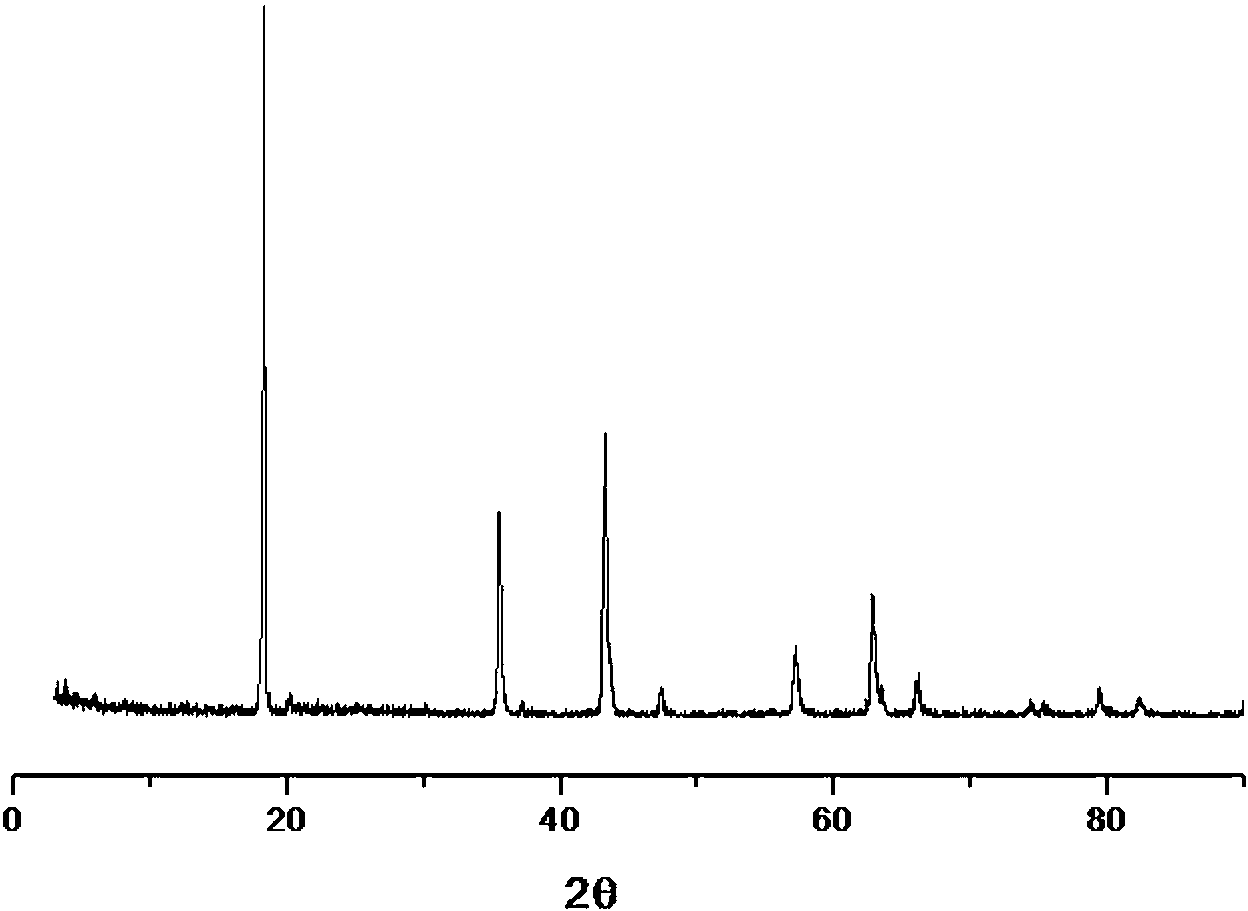
Image
Examples
Embodiment 1
[0027] Commercially available 0.1~0.2 μm TiO 2 Powder and TiO 2 After mixing 10% glucose evenly, take 2g, press it into a test piece with a diameter of 20mm and a thickness of 3mm under 8MPa, and sinter at 600°C for 3 hours in an argon atmosphere to obtain a carbon source-coated TiO 2 Audition. After the test piece was tightly wrapped with molybdenum mesh, a thin molybdenum wire was wound on a long molybdenum wire as the cathode, a graphite rod was used as the anode, and a mixture of LiCl and NaCl with a melting molar ratio of 2:1 was used as the electrolyte. In the middle, the temperature is 500°C, the voltage is 3.2V, and the electrolysis is performed for 5 hours. After the electrolysis is completed, the cathode is raised from the molten salt liquid level and cooled to room temperature in the electrolytic cell, and the product is taken out, washed with distilled water, and vacuum dried at 60°C. , burned in the air at 750°C for 2 hours, and the resulting product was about 2...
Embodiment 2
[0029] Commercially available 0.1~0.2 μm TiO 2 Powder and TiO 2 After mixing 10% acetylene black evenly, take 5g, press under 5MPa to make two test pieces with a diameter of 20mm and a thickness of 5mm, and sinter at 600°C for 2 hours in an argon atmosphere. After the test piece is tightly wrapped with stainless steel mesh, stainless steel wire is wound on the long stainless steel wire as cathode, graphite rod is used as anode, and a mixture of LiCl and KCl with a melting molar ratio of 1:1 is used as electrolyte, in an argon atmosphere , the temperature is 600°C, the voltage is 2.8V, electrolysis for 4 hours, after the electrolysis is completed, the cathode is raised from the molten salt liquid level and cooled to room temperature in the electrolytic cell, the product is taken out, washed with distilled water, and vacuum dried at 60°C, Burned at 600°C in air for 3 hours to obtain 5.1g Li 4 Ti 5 o 12 .
Embodiment 3
[0031] Commercially available 0.1~0.2 μm TiO 2 with TiO 2 After mixing 30% activated carbon evenly, take 0.5 g, press it under 10 MPa to form a test piece with a diameter of 5 mm and a thickness of 1 mm, and sinter at 600 ° C for 3 hours in an argon atmosphere. Wind the test piece with molybdenum wire to make a cathode, use a graphite rod as an anode, and use a mixture of LiCl, KCl and NaCl with a melting molar ratio of 1:1:1 as an electrolyte. In an argon atmosphere, the temperature is 400 °C and the voltage is 3.2V, electrolysis for 5 hours, after the electrolysis is completed, lift the cathode out of the molten salt liquid level and cool it to room temperature in the electrolytic cell, take out the product, wash it with distilled water, dry it in vacuum at 60°C, and burn it in the air at 850°C for 1 hours, to get 0.4g Li 4 Ti 5 o 12 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com