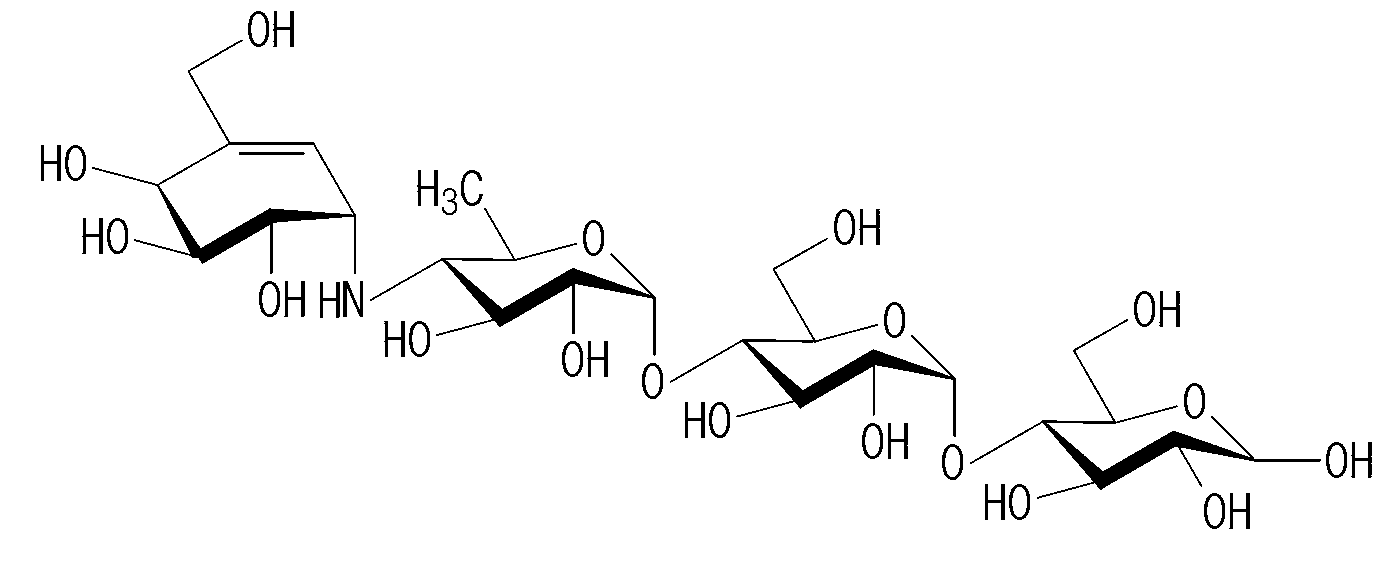
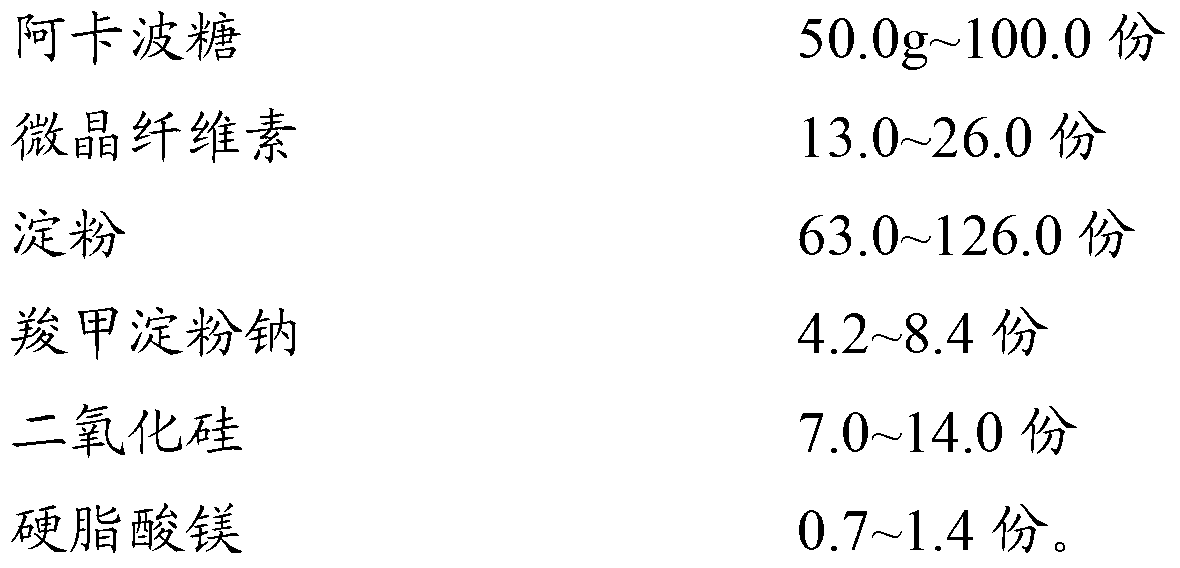Acarbose tablets and preparation method thereof
A technology of acarbose and acarbose, which can be used in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. Eligibility, poor fluidity of acarbose, etc., to achieve the effect of stable and controllable product quality, control of disintegration degree and microbial limit, and ease of subsequent processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040]Acarbose 50.0g Microcrystalline Cellulose 13.0 Starch 63.0g
[0041] Sodium starch glycolate 4.2g Silicon dioxide 7.0g Magnesium stearate 0.7g
[0042] Acarbose, microcrystalline cellulose, starch, sodium starch glycolate, silicon dioxide, and magnesium stearate were respectively pulverized through a 100-mesh sieve, and set aside.
[0043] Put acarbose, microcrystalline cellulose, starch and 2 / 3 carboxymethyl starch sodium into the mixer, start the machine and mix evenly to obtain a premix; put the above premix into GZL series dry roller compaction granules In the machine, the roller pressure is 40 Pa, the pressing roller speed is 20 rpm, and the mixture transmission speed is 60 rpm, start the machine, pass through a 20-mesh sieve after granulation, and then get the granules; mix the above granules with stearic acid Magnesium, silicon dioxide, and the remaining sodium starch glycolate were added into a multidirectional mixer and mixed for 30 minutes, and the resulting m...
Embodiment 2
[0045] Acarbose 80.0g Microcrystalline Cellulose 18.0g Starch 94.0g
[0046] Sodium starch glycolate 6.3g Silicon dioxide 10.5g Magnesium stearate 1.05g
[0047] Acarbose, microcrystalline cellulose, starch, sodium starch glycolate, silicon dioxide, and magnesium stearate were respectively pulverized through a 100-mesh sieve, and set aside.
[0048] Put acarbose, microcrystalline cellulose, starch and 2 / 3 carboxymethyl starch sodium into the mixer, start the machine and mix evenly to obtain a premix; put the above premix into GZL series dry roller compaction granules In the machine, the roller pressure is 20 Pa, the pressing roller speed is 17 rpm, and the mixture transmission speed is 45 rpm, the machine is started, and the granules are discharged through a 20-mesh sieve to obtain granules; the above granules are mixed with stearic acid Magnesium, silicon dioxide, and the remaining sodium starch glycolate were added into a multidirectional mixer and mixed for 30 minutes, and...
Embodiment 3
[0050] Acarbose 100.0g Microcrystalline Cellulose 26.0g Starch 126.0g
[0051] Sodium starch glycolate 8.4g Silicon dioxide 14g Magnesium stearate 1.4g
[0052] Acarbose, microcrystalline cellulose, starch, sodium starch glycolate, silicon dioxide, and magnesium stearate were respectively pulverized through a 100-mesh sieve, and set aside.
[0053] Put acarbose, microcrystalline cellulose, starch and 2 / 3 carboxymethyl starch sodium into the mixer, start the machine and mix evenly to obtain a premix; put the above premix into GZL series dry roller compaction granules In the machine, the roller pressure is 50 Pa, the pressing roller speed is 22 rpm, and the mixture transmission speed is 80 rpm, start the machine, pass through a 20-mesh sieve after granulation, and then get the granules; mix the above granules with stearic acid Magnesium, silicon dioxide, and the remaining sodium starch glycolate were added into a multidirectional mixer and mixed for 30 minutes, and the resultin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com