Application of p-hydroxyl acetophenone glycosidase to medicine for autoimmunity disease and transplant rejection disease
A para-hydroxyacetophenone, autoimmune disease technology, applied in metabolic diseases, skin diseases, bone diseases and other directions, can solve the problems of increasing the economic burden of patients, non-specific cells, adverse reactions, etc., to reduce the economic burden of patients, inhibit Occurrence and development, rich sources of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: Preparation of p-hydroxyacetophenone glucoside
[0037]Rabbit wind (Incarvillea delavay) whole herb 30kg, reflux extraction with 80% ethanol 2L each time for 3 times, each time for 2 hours, combined extracts, concentrated under reduced pressure to obtain 600g extract, diluted with 3L of water, adjusted with 2% HCl pH to 2-3, filter to obtain filtrate and filter residue, add 20% NaOH to the filtrate to adjust pH to 11, extract with chloroform three times, 2 L each time, to obtain chloroform fraction; concentrate the chloroform extraction fraction under reduced pressure to obtain 250 g of crude product. Mix the crude product with 500g of silica gel (200-300 mesh) to mix the sample, elute with chloroform-acetone (20:1-2:1) system gradient, and collect p-hydroxyacetophenone glucoside (detected by thin-layer chromatography) Fractions were subjected to C18 reverse-phase silica gel (40-70 μm) column chromatography, gradient elution with methanol-water (50%-80%), ...
Embodiment 2
[0038] Example 2 In vitro culture of human dendritic cells and treatment of DCs with p-hydroxyacetophenone glucoside
[0039] 50 mL / person of peripheral blood from healthy adults was extracted intravenously, anticoagulated with 1% sterile heparin, and density gradient centrifugation was used (2000r / min for 20min, carefully absorb the white cloudy mononuclear cells in the interface layer, and 2000r / min was used in a 15mL centrifuge tube) Centrifuge once, centrifuge once at 1500r / min, centrifuge once at 1000r / min, separate the peripheral blood mononuclear cells, suspend the cells with RPMI1640 (1640 medium) containing 10% calf serum, adjust the cell concentration to 2×10 6 / mL, add to 24-well culture plate, 0.5mL / well, 37℃, 5%CO 2 The incubator was cultured for 2 hours to make the monocytes adhere to the wall, and the culture plate was lightly washed with warm serum-free RPMI1640 to remove the adherent cells, so as to obtain the adherent monocytes. Add serum-containing RPMI1640...
Embodiment 3
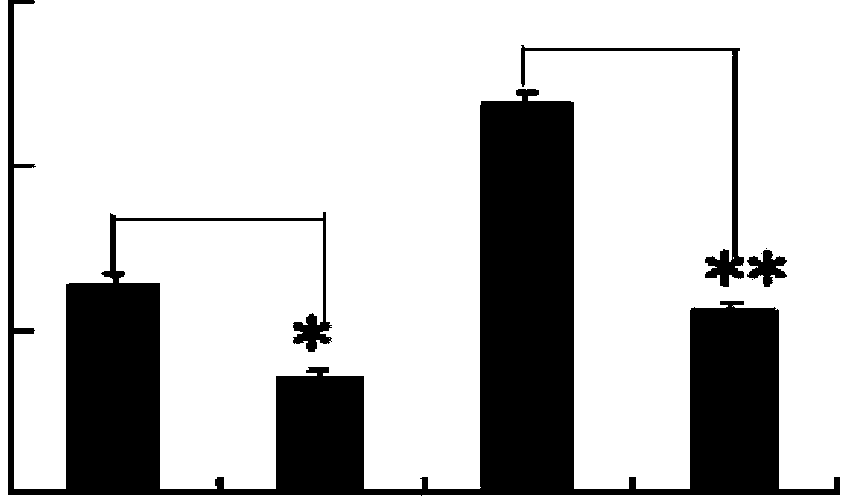
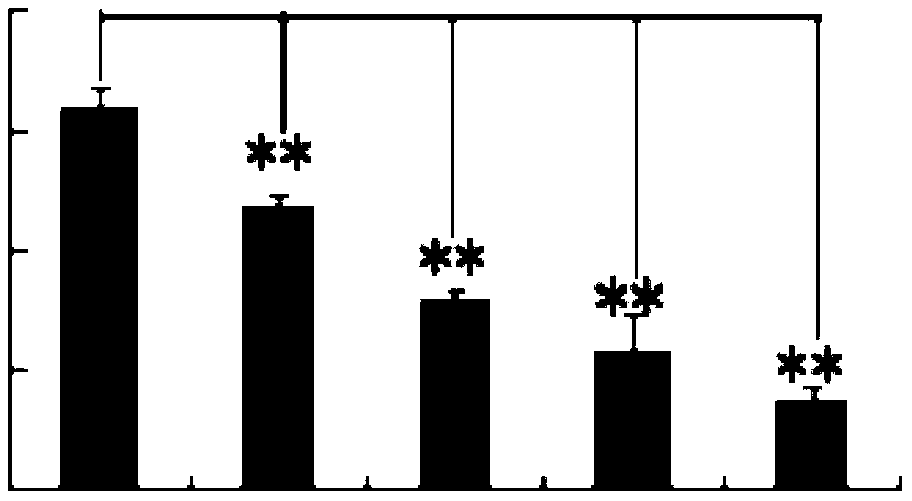
[0041] Example 3 Effect of p-Hydroxyacetophenone Glucoside on Human Dendritic Cells Secreting IL-10
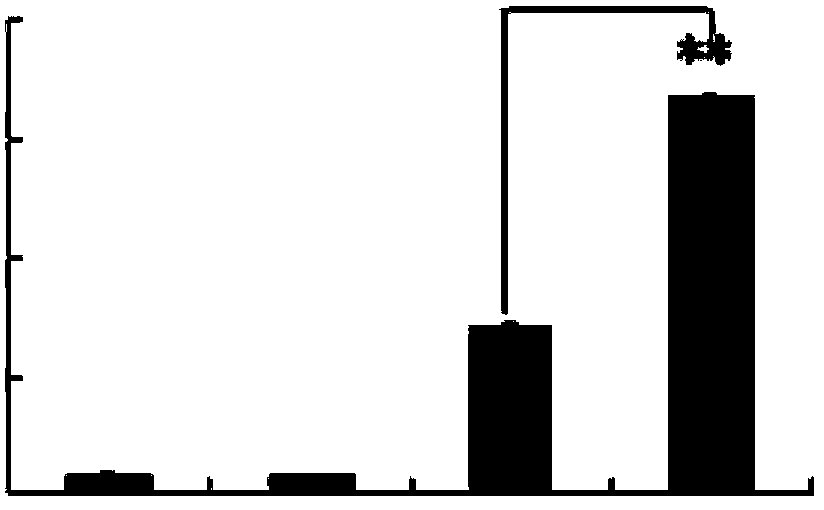
[0042] Dendritic cells of p-hydroxyacetophenone glucoside treatment group and untreated group cultured according to the method of Example 2, supernatants were collected, and IL-10 was detected with an ELISA (enzyme-linked immunosorbent assay) kit for IL-10. 10 content. The concentration of IL-10 secreted by monocyte-transformed dendritic cells in the p-hydroxyacetophenone glucoside treatment group and the untreated group was 435±22pg / mL and 92±14pg / mL, and there was a significant difference (Pfigure 1 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com