Antimicrobial peptide MP1102 of anti-drug resistance staphylococcus aureus and preparation method and application thereof
A technology of MP1102 and A-MP1102, applied in botany equipment and methods, microorganism-based methods, biochemical equipment and methods, etc., can solve the problems of high production cost, insufficient antibacterial activity, and low yield of gene cloning expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Example 1 Antimicrobial Peptide MP1102 Sequence Design
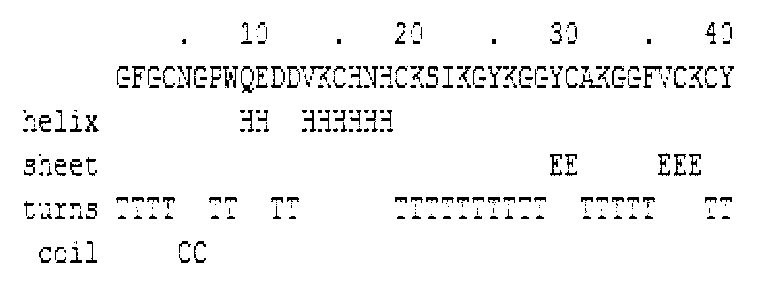
[0061] The physical and chemical parameters of NZ2114 series derivative peptides were statistically analyzed by bioinformatics tools, and the structural parameters of NZ2114 were optimized in combination with the structure and activity of antimicrobial peptides to reduce the instability coefficient and fat cluster coefficient of NZ2114, increase the hydrophobic moment of NZ2114, and increase α According to the helicity parameters, the 9th, 13th, and 14th amino acids of NZ2114 were subjected to site-directed mutations to design a new antimicrobial peptide MP1102. The primary amino acid sequence of MP1102 was: GFGCNGPWQEDDVKCHNHCKSIKGYKGGYCAKGGFVCKCY.
[0062] The primary sequence result parameters are shown in Table 2:
[0063] Table 2 Analysis of structural parameters of antimicrobial peptides
[0064]
[0065] The secondary structure was analyzed by bioinformatics software (http: / / emboss.bioinformatics.nl / ), ...
Embodiment 2
[0066] Example 2 Acquisition of the gene encoding the antimicrobial peptide MP1102
[0067] (1) Optimization of the MP1102 gene expression sequence encoding the antimicrobial peptide MP1102
[0068] According to the yeast codon table Pichia pastoris[gbpln]:137CDS's(81301codons)
[0069]
[0070]
[0071]
[0072] The DNA sequence after codon optimization is shown in SEQ ID No:2. Sequence feature: 120bp; type: nucleic acid; chain type: double-stranded; topology: linear; molecular type: double-stranded DNA.
[0073] (2) Design of gene expression cassette
[0074] Based on effectively terminating the translational expression of MP1102, two consecutive TAA stop codons were inserted at the C-terminus of the MP1102 coding sequence. The signal peptide cleavage site kex2 site was inserted into the N-terminus of MP1102 to realize the natural secretion and expression of MP1102 in Pichia pastoris. XhoI was designed at both ends of the MP1102 gene, XbaI endonuclease sites Xho...
Embodiment 3
[0091] Example 3 Recombinant Yeast Expression Vector Construction
[0092] (1) The MP1102 gene obtained in Example 2 and the pPICZαA vector were double digested with XhoI and XbaI endonucleases.
[0093] The double enzyme digestion system is as follows:
[0094]
[0095] Digestion conditions: 37°C, 4h in water bath.
[0096] The digested product was recovered with a DNA product recovery kit (purchased from Tiangen Biological Co., Ltd.), and stored at -20°C for future use.
[0097] After the MP1102 gene and the pPICZαA vector were digested with XbaI and XhoI, the MP1102 gene was connected to the linearized pPICZαA vector with T4 DNA ligase. The connection system was as follows:
[0098]
[0099] Connection conditions: 25°C, 1h.
[0100] (2) Transform the recombinant vector in (1) into Escherichia coli DH5α, and the specific operation steps are as follows: Freeze and store Escherichia coli DH5α competent cells at -80°C, and thaw immediately on ice. Take 90 μL of compet...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com