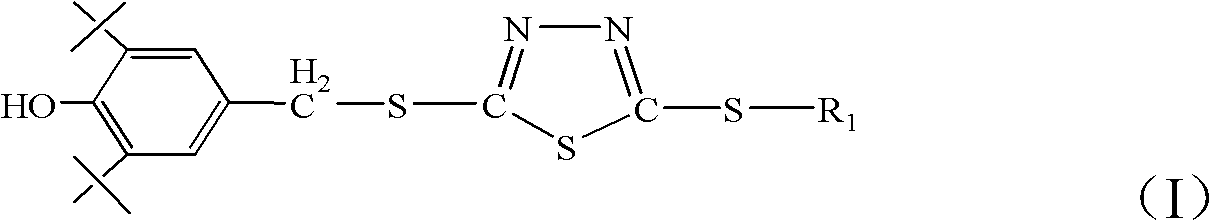
Screen phenol-containing thiadiazole type antioxygen antiwear additive and preparation method thereof
A thiadiazole type, shielding phenol technology, applied in the direction of additives, petroleum industry, organic chemistry, etc., to achieve good solubility and excellent anti-oxidation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Add 15.0g 2,5-dimercapto-1,3,4-thiadiazole (0.1mol) and 80ml ethyl acetate into a 500ml four-neck flask equipped with a reflux condenser and a thermometer, stir to dissolve, and Add 20mL of 4.0g NaOH (0.1mol) aqueous solution dropwise for 10min, then add 0.3g tetrabutylammonium bromide (0.001mol) as a catalyst, add 13.7g (0.1mol) bromobutyl at 20°C dropwise Alkanes, the time for the dropwise addition is 30 minutes. After the dropwise addition, gradually raise the temperature to 76°C for reflux reaction for 4 hours. After the reaction, cool to room temperature and filter to obtain a pale yellow solid powder , 4-thiadiazole.
Embodiment 2
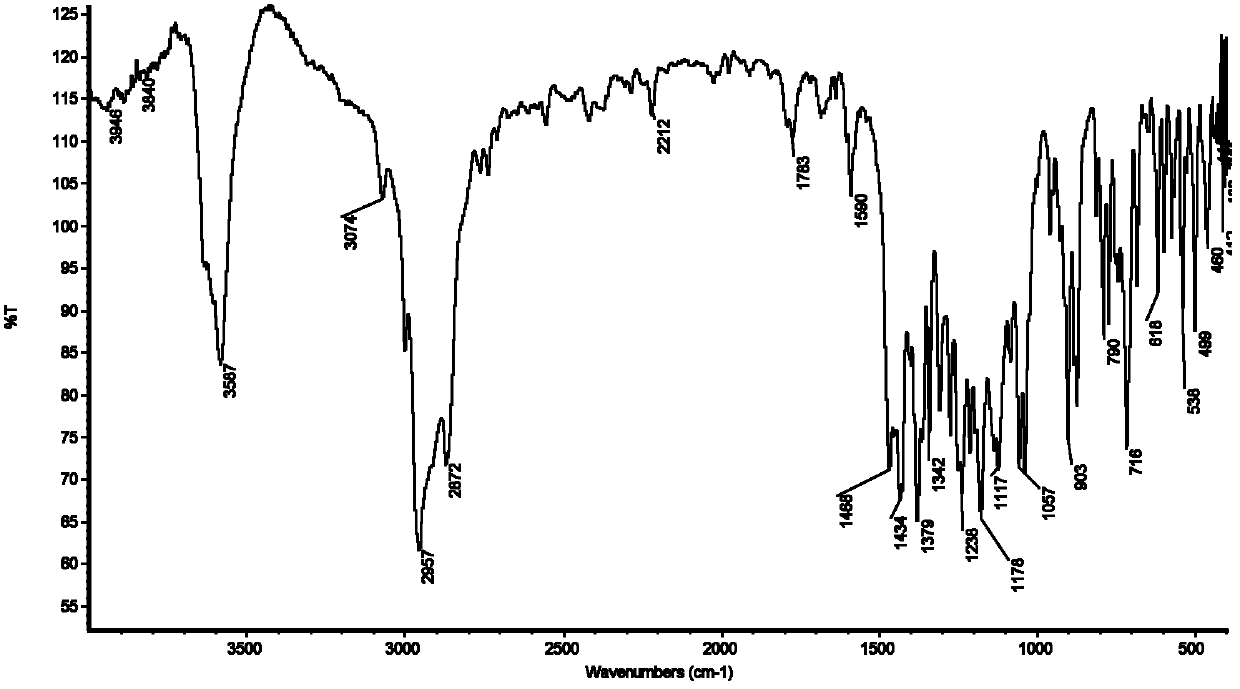
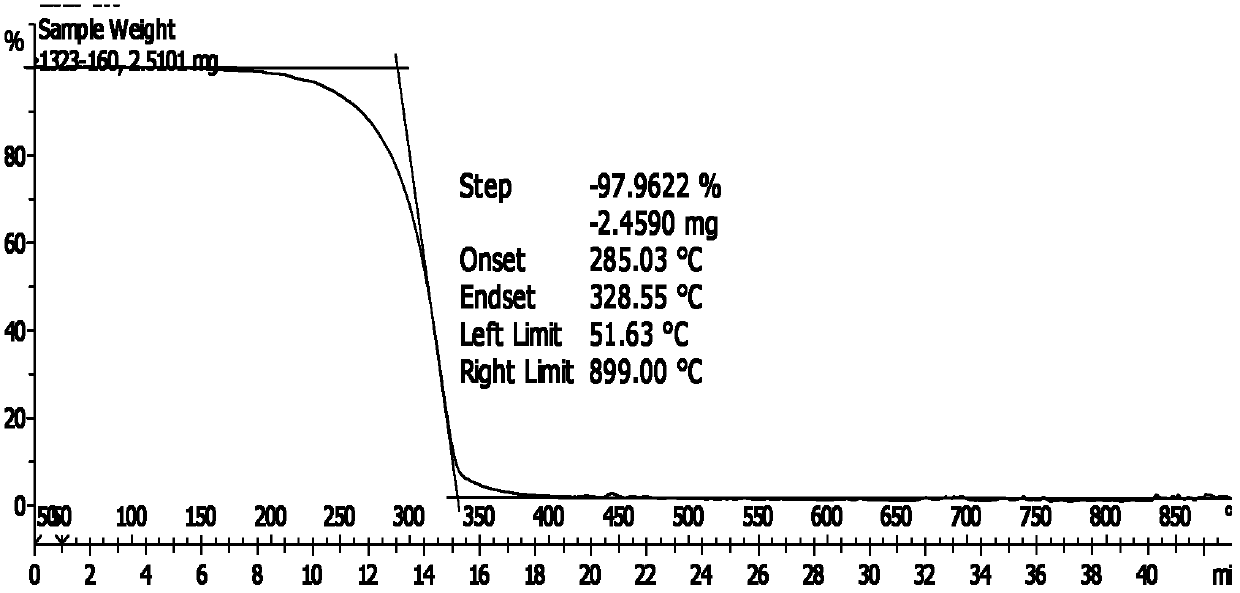
[0044] Add 20.5g 2-thiobutyl-5-mercapto-1,3,4-thiadiazole (0.1mol) synthesized by Example 1 in a 500ml four-necked flask equipped with a reflux condenser and a thermometer, while mixing and adding 20.6 g 2,6-di-tert-butylphenol (0.1mol), 3.6g paraformaldehyde (0.12mol), carry out stirring reaction with 75ml N,N-dimethylformamide as solvent, heat up to 120°C and add catalyst two n Butylamine 0.65g (0.005mol) was refluxed at 120-130°C for 5 hours. After the reaction, the product was diluted with a mixed solvent of petroleum ether and tetrahydrofuran, poured out and washed with water until neutral, dried with anhydrous sodium sulfate, and evaporated. The solvent was removed to obtain the product 2-(3,5-di-tert-butyl-4-hydroxy-benzyl)thio-5-butylthio-1,3,4-thiadiazole. The S content of the product is 21.69%, and the theoretical S content is 22.14%; the N content of the product is 6.56%, and the theoretical N content is 6.60%; the melting point of the product is 90°C. figure 1 It ...
Embodiment 3
[0046] Add 15.0g of 2,5-dimercapto-1,3,4-thiadiazole (0.1mol) and 80ml of ethanol into a 500ml four-necked flask equipped with a reflux condenser and a thermometer, stir to dissolve, and add dropwise at room temperature at 20°C 4.4g NaOH (0.11mol) aqueous solution 21mL, dropwise time is 20min, then add 0.3g tetrabutylammonium bromide (0.001mol) as a catalyst, add dropwise 14.9g (0.1mol) chlorooctane at 20°C, The dropwise addition time is 40min. After the dropwise addition, gradually raise the temperature to 76°C for reflux reaction for 6h. After the reaction, cool to room temperature and filter to obtain light yellow solid powder 2-thiooctyl-5-mercapto-1,3,4 - Thiadiazoles.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com