A kind of preparation method of Bacillus subtilis C3 and anti-Listeria monocytogenes bacteriocin
A technology of Bacillus subtilis and Listeria monocytogenes, which is applied in the field of microbiology, can solve problems such as no related reports, and achieve the effect of simple extraction process, wide sources and efficient purification method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1, the preparation of Bacillus subtilis C3 bacteriocin crude extract
[0026] 1. Preparation of Bacillus subtilis C3 fermentation broth
[0027] (1) Preparation of seed solution
[0028] Seed medium formula (W / V, mass-volume ratio concentration): glucose 1%, peptone 1%, NaCl 1%, KH 2 PO 4 0.15%, MgS0 4 ·7H 2 O0.15%, pH natural;
[0029] Seed culture conditions: The activated Bacillus subtilis was transferred into the seed medium at 1% inoculum amount, the culture temperature was 37°C, the liquid volume was 75mL / 250mL, and the shaker was shaken at 150r / min for 18h, and the number of viable bacteria was about 10. 9 CFU / mL.
[0030] (2) Preparation of fermentation broth
[0031] Fermentation medium formula (W / V, mass-volume ratio concentration): glucose 1%, peptone 1.5%, yeast extract powder 1.5%, KH 2 PO 4 0.1%, MgSO 4 ·7H 2 O0.15%, NaCl0.5%, pH7.0;
[0032] Fermentation conditions: The seed liquid was transferred into the fermentation medium at 2% ...
Embodiment 2
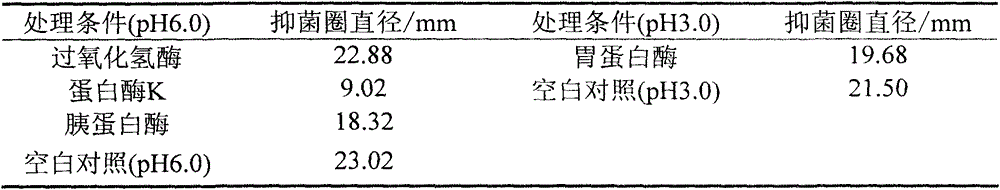
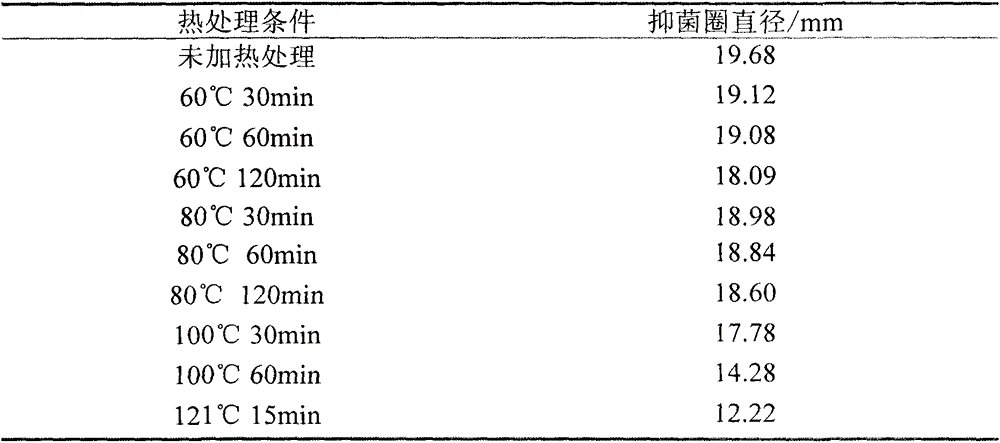
[0036] Example 2. Bacteriostatic properties and physicochemical properties of the crude extract of Bacillus subtilis C3 bacteriocin
[0037] During the experiment, the antibacterial and physicochemical properties of the crude extract of C3 bacteriocin were determined by the tube-plate method.
[0038] Tube-dish method: first dilute the indicator strain to 10 7 CFU / mL, after mixing with the corresponding solid medium that has been heated and melted, pour about 15 mL into a petri dish. After solidification, gently place a sterile Oxford cup. Take 100 μL of Bacillus subtilis C3 crude extract and add it to the Oxford cup and culture at 37 °C. 12h, the inhibition zone was observed, and the diameter of the inhibition zone was measured with a vernier caliper, and the reading was accurate to 0.01 mm.
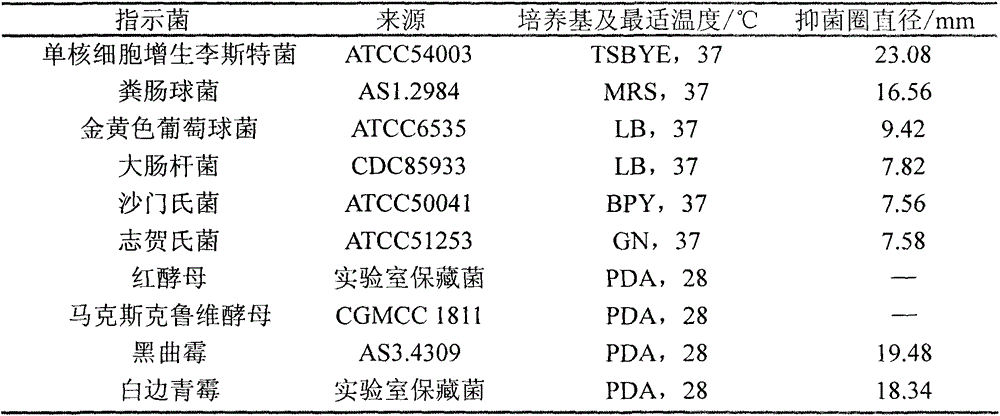
[0039] 1. Antibacterial properties of crude extract of Bacillus subtilis C3 bacteriocin
[0040] The bacteriostatic effect of table 1 Bacillus subtilis C3 bacteriocin crude extract
...
Embodiment 3
[0062] Embodiment 3, Bacillus subtilis C3 bacteriocin purification method
[0063] 1. Purification of the crude extract of Bacillus subtilis C3 bacteriocin
[0064] (1) Step-by-step elution and purification by anion exchange chromatography
[0065] The crude extract of Bacillus subtilis C3 bacteriocin was filtered and sterilized with a 0.2 μm sterile filter, and was eluted and purified by DEAE-Sepharose Fast Flow anion exchange chromatography step by step. The column size was 10 mm × 200 mm; buffer: A Solution 0.025mol / L pH7.5Tric-HCl, solution B contains 0.4mol / L NaCl in 0.025mol / L pH7.5Tric-HCl; step-by-step elution steps: 50min 100% solution A→90min 70% solution A+30% solution B →40min50%A solution+50%B solution→40min100%B solution; the flow rate is 1mL / min, the sample volume is 5mL, the automatic collector is 5mL / tube, and the tube plate method is used to detect the effluent of the collected eluate for Listeria monocytogenes. Antibacterial activity, the test showed that ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com