Optically-active diazabicyclooctane derivative and method for manufacturing same
A diazacyclic, optically active technology, applied in the field of optically active diazacyclooctane derivatives, can solve the problem of fewer candidates for development
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
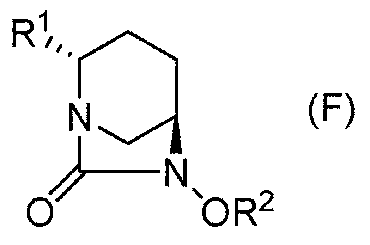
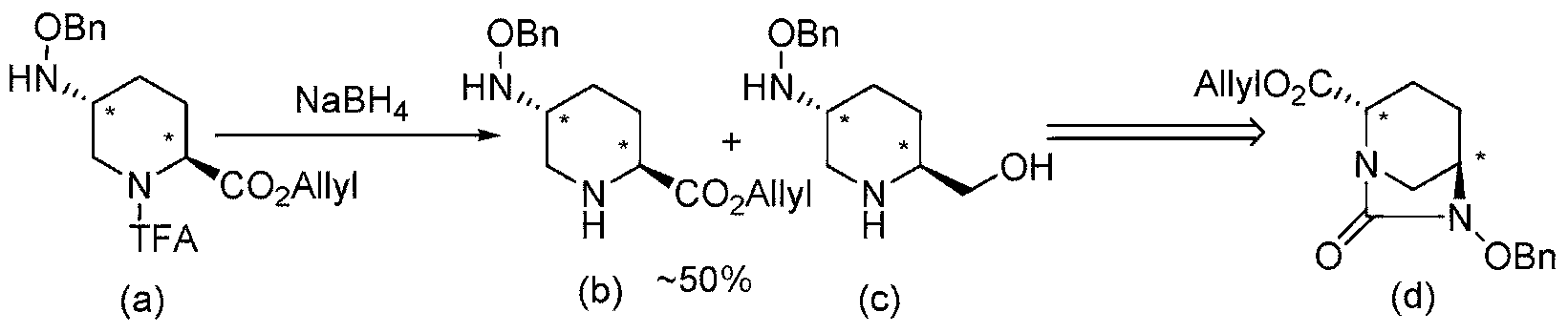
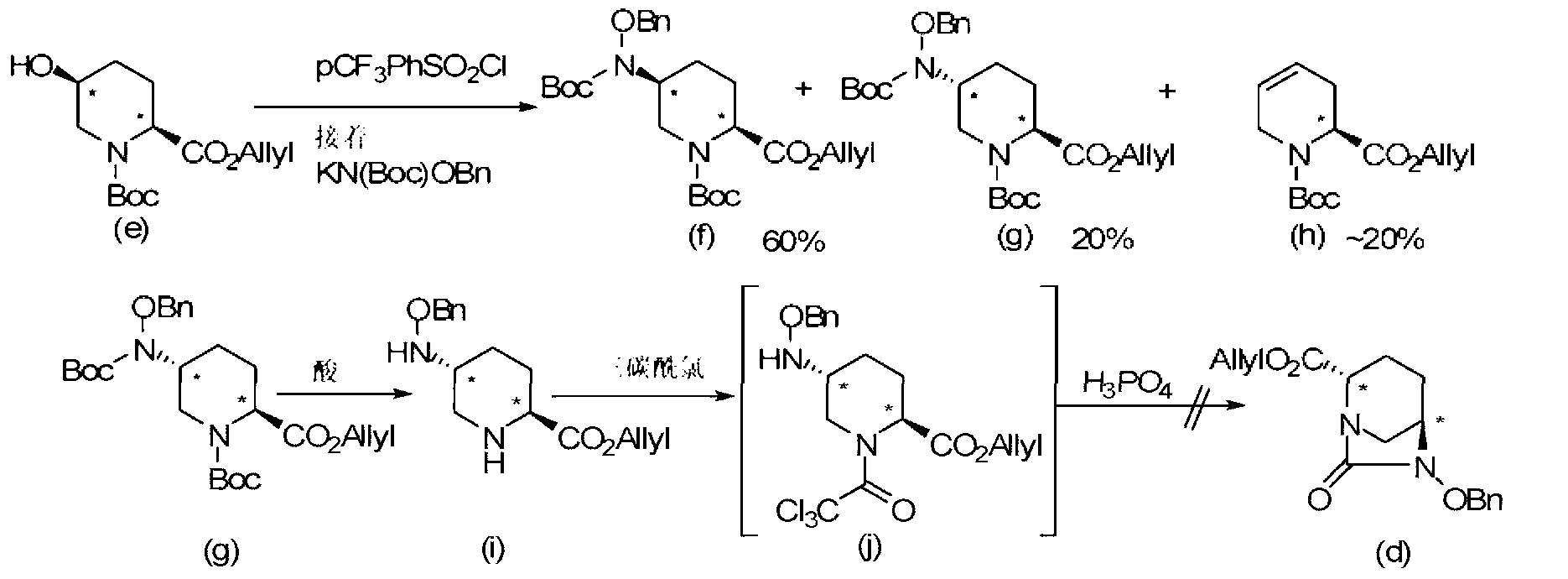
[0121] Among the compounds represented by the formula (F) of the present invention obtained by the above-mentioned production method of the present invention, the following formulas (F1), (F1-3a), (F1-3b), (F1-1a), (2S,5R)-6-(benzyloxy)-7-oxo-1,6-diazacyclo[3.2.1]octane-2- represented by (F1-2) and (F1-4) tert-butyl carboxylate, (2S,5R)-6-(benzyloxy)-7-oxo-1,6-diazacyclo[3.2.1]octane-2-carboxylate methyl ester, (2S,5R)-6-(Benzyloxy)-7-oxo-1,6-diazacyclo[3.2.1]octane-2-carboxylic acid allyl ester, (2S,5R)- Cyclohexylammonium salt of 6-(benzyloxy)-7-oxo-1,6-diazacyclo[3.2.1]octane-2-carboxylic acid, (2S,5R)-6-(benzyloxy base)-7-oxo-1,6-diazacyclo[3.2.1]octane-2-carboxylic acid and (2S,5R)-6-(benzyloxy)-7-oxo-1, 6-diazacyclo[3.2.1]octane-2-carboxamide can be obtained in the form of crystals of optically active diazacyclooctane derivatives, so it has the advantages of easy separation, purification, storage and transportation . This shows that the present invention is an indust...
Embodiment 1
[0413] (2S,5S)-5-Hydroxypiperidine-2-carboxylic acid tert-butyl ester (B)
[0414] [chemical formula 46]
[0415]
[0416] Add 10% palladium carbon ( moisture about 50%) 10.1 g, stirred vigorously at room temperature overnight under a hydrogen atmosphere. The catalyst mixture was filtered through celite, and the filtrate was concentrated to obtain 39.3 g of the title compound as a colorless solid (97% yield). The excess rate of optically active substances is above 99%ee (CHIRALPAK AD-H, 4.6×150mm, UV210nm, diethylamine / hexane / ethanol=0.1 / 80 / 20, flow rate 1mL / min, retention time 6.3min).
[0417] [α] 20 D -28.7°(c1.01, CHCl 3 ); 1 H NMR (400MHz, CDCl 3 ,δ):1.47(s,9H),1.63(m,1H),1.79-1.84(m,3H),2.82(dd,J=12.2,2.2Hz,1H),3.02(ddd,J=12.2,3.7 ,1.7Hz,1H),3.21(m,1H),3.80(m,1H);MS m / z:202(M+1).
Embodiment 2
[0419](2S,5S)-5-Hydroxy-1-(2,2,2-trifluoroacetyl)piperidine-2-carboxylic acid tert-butyl ester (C)
[0420] [chemical formula 47]
[0421]
[0422] Under argon atmosphere, the solution of 39.14 g (194 mmol) of (2S,5S)-5-hydroxypiperidine-2-carboxylic acid tert-butyl ester in anhydrous tetrahydrofuran (450 mL) was cooled to -3~-5 °C, and three 78.7 g (776 mmol) of ethylamine, and 81.5 g (388 mmol) of trifluoroacetic anhydride were added dropwise over 30 minutes. The reaction mixture was reacted at -3~-5°C for 1 hour, water (90 mL) was added, the temperature was raised to room temperature, and stirred for 1 hour. Water (740mL) was added to the reaction mixture, extracted with ethyl acetate (450mL×3 times), and the combined organic layer was sequentially washed with 5% aqueous citric acid (450mL), 6.5% aqueous sodium bicarbonate (450mL) and water ( 450mL) for washing. The residue obtained by distilling off the solvent under reduced pressure was subjected to silica gel colum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com