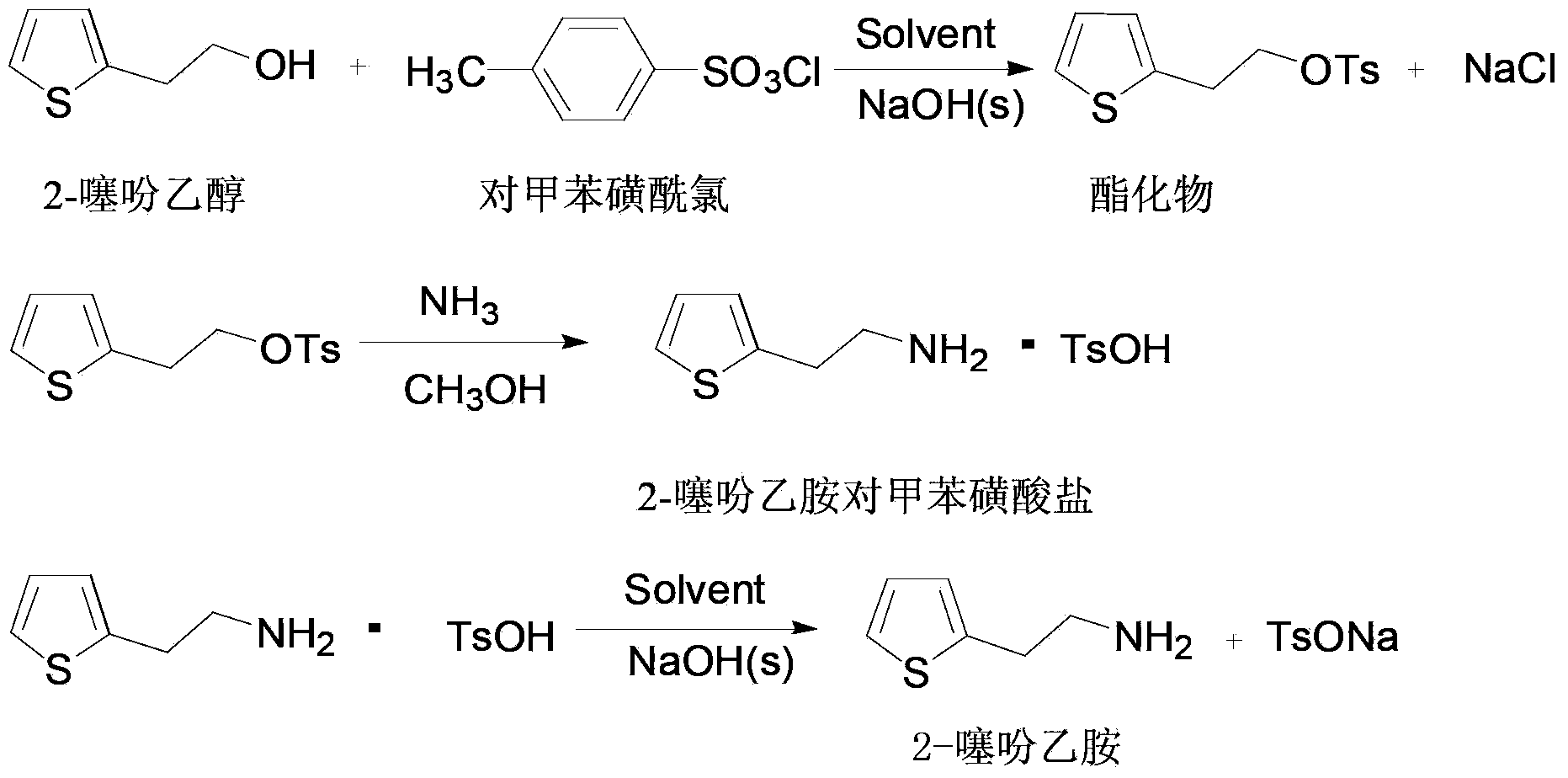
Synthetic method of 2-thiophene ethylamine
A technology of thienylethylamine and its synthesis method, which is applied in the fields of medicine and chemical industry, can solve the problems of 2-thienylethylamine product handling, cumbersome product post-processing, a large amount of waste water and waste acid, etc., achieve mild reaction conditions and reduce production costs , the effect of high total yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1: the preparation of esterification product:
[0027] Add 35.0g (0.184mol) p-toluenesulfonyl chloride and 45ml toluene to a 250ml four-neck flask equipped with a drying tube at room temperature, stir and dissolve, then add 22.5g (0.176mol) thiophene ethanol, cool to 0-5°C, Add 8.5g (0.213mol) of sodium hydroxide, stir and react at 0-5°C for 5 hours, suction filter after the reaction, rinse the filter cake with 30ml of toluene, and drain to obtain a wet product of solid sodium chloride and sodium hydroxide at about 12.9 g. The filtrate was decompressed to remove toluene, until there was no flow, and then distilled under reduced pressure for 30 minutes to obtain 49.8 g of sulfonic acid ester concentrate. The high performance liquid chromatography (HPLC) analysis showed that the content of sulfonic acid ester was 99.4%, and the yield was 99%. .
Embodiment 2
[0028] Embodiment 2: the preparation of esterification product:
[0029] Add 35.0g (0.184mol) p-toluenesulfonyl chloride and 45ml toluene to a 250ml four-neck flask equipped with a drying tube at room temperature, stir and dissolve, then add 22.5g (0.176mol) thiophene ethanol, cool to 0-5°C, Add 14.4g (0.360mol) of sodium hydroxide, stir and react at 0-5°C for 5 hours, filter with suction after the reaction, rinse the filter cake with 30ml of toluene, and drain to obtain a wet product of solid sodium chloride and sodium hydroxide of about 20.1 g. The filtrate was decompressed to remove toluene, until there was no flow, and then distilled under reduced pressure for 30 minutes to obtain 50.0 g of sulfonic acid ester concentrate. The high performance liquid chromatography (HPLC) analysis showed that the content of sulfonic acid ester was 99.0%, and the yield was 99%. .
Embodiment 3
[0030] Embodiment 3: the preparation of esterification product:
[0031] Add 35.0g (0.184mol) p-toluenesulfonyl chloride and 45ml toluene to a 250ml four-necked flask equipped with a drying tube at room temperature, stir and dissolve, add 22.5g (0.176mol) thiophenethanol, and add 8.5g ( 0.213mol) sodium hydroxide, stirred and reacted at 25°C for 5 hours, suction filtered after the reaction, the filter cake was rinsed with 30ml of toluene, and dried to obtain about 12.9g of wet solid sodium chloride and sodium hydroxide. The filtrate was decompressed to remove toluene, until there was no flow, and then distilled under reduced pressure for 30 minutes to obtain 49.6 g of sulfonic acid ester concentrate. The high performance liquid chromatography (HPLC) analysis showed that the content of sulfonic acid ester was 98.6%, and the yield was 98%. .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com