A kind of method that catalytic oxidation reaction prepares dibasic carboxylic acid
A technology of catalytic oxidation and dibasic carboxylic acid, which is applied in the direction of oxidative preparation of carboxylic acid, chemical industry, organic chemistry, etc. It can solve the problems of heteropolyacid catalyst unstable properties, short service life, small specific surface, etc., and achieve the purpose of inhibiting the product Effects of side reactions, easy addition and removal, and prevention of thermal decomposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] This example illustrates the direct oxidation of cyclohexanone to adipic acid.
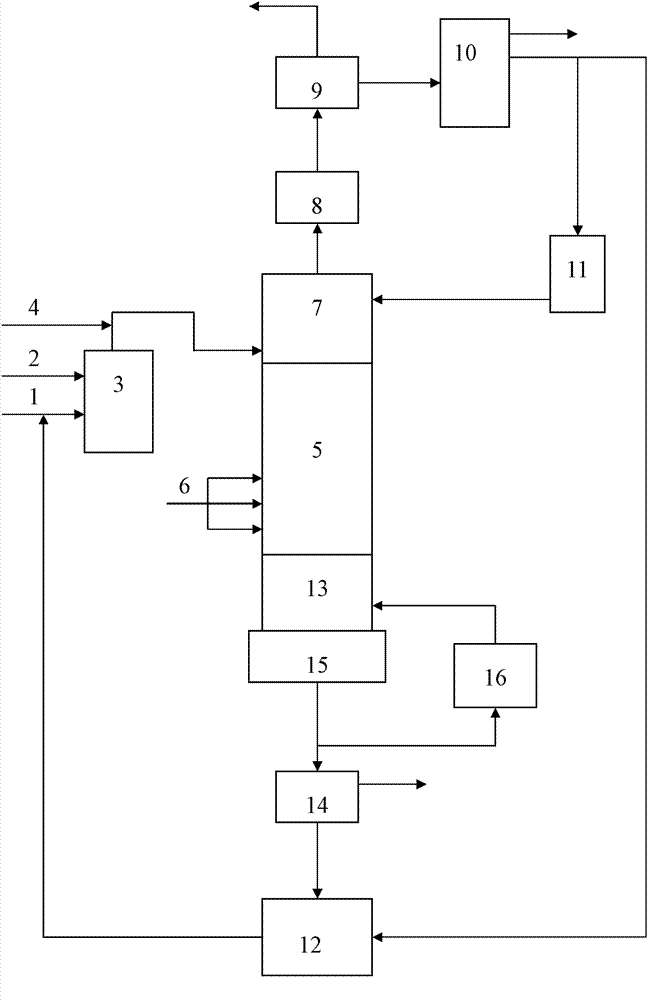
[0038] The reaction tower is a sieve plate tower, the catalyst used is HTS, and the catalyst particle diameter distribution is 1-200 μm; the reaction raw materials are 30% hydrogen peroxide and more than 99.9% cyclohexanone; the solvent is acetone. Prepare the acetone slurry with a catalyst concentration of 5% in the catalyst storage tank, and fully stir and disperse it for later use. Use acetone to test the device under the set conditions. After the device is stable, mix the catalyst slurry, hydrogen peroxide, and cyclohexanone Add to the reaction section in the tower. The absolute pressure is 0.23MPa, the temperature of the reaction section is 110°C, the molar ratio of cyclohexanone to acetone in the reaction section is 1:5, and the mass space velocity of cyclohexanone is 1.5h -1 , The reflux ratio of the top material is 6:1. The feed molar ratio of cyclohexanone and hydrogen peroxide i...
Embodiment 2
[0040] This example illustrates the direct oxidation of cyclohexanone to adipic acid.
[0041] The reaction tower is a sieve plate tower, and the catalyst used is HTS spray-molded with silica sol as a binder. The content of HTS is 50%, and the particle diameter distribution of the catalyst is 40-600 μm; Hexanone; solvent is acetone. Prepare the acetone slurry with a catalyst concentration of 15% in the catalyst storage tank, and fully stir and disperse it for standby. Use acetone to test run the device under the set conditions. After the device is stable, mix the catalyst slurry, hydrogen peroxide, and cyclohexanone Add to the reaction section in the tower. The absolute pressure is 0.20MPa, the temperature of the reaction section is 108°C, the molar ratio of cyclohexanone to acetone in the reaction section is 1:7, and the mass space velocity of cyclohexanone is 1.5h -1 , The reflux ratio of the top material is 8:1. The feed molar ratio of cyclohexanone and hydrogen peroxide...
Embodiment 3
[0043] This example illustrates the direct oxidation of cyclopentane to glutaric acid.
[0044] The reaction tower is a sieve plate tower, the catalyst used is HTS, and the catalyst particle diameter distribution is 1-200 μm; the reaction raw materials are 30% hydrogen peroxide and more than 99.9% cyclopentanone; the solvent is acetone. Prepare the acetone slurry with a catalyst concentration of 5% in the catalyst storage tank, and fully stir and disperse it for later use. Use acetone to test the device under the set conditions. After the device is stable, mix the catalyst slurry, hydrogen peroxide, and cyclopentanone. Add to the reaction section in the tower. The absolute pressure is 0.19MPa, the temperature of the reaction section is 108°C, the molar ratio of cyclopentane to acetone in the reaction section is 1:5, and the mass space velocity of cyclopentane is 1.5h -1 , The reflux ratio of the top material is 6:1. The feed molar ratio of cyclopentane to hydrogen peroxide i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com