Sodium picosulfate enteric-coated tablet and preparation method thereof
A technology of sodium picosulfate and enteric-coated tablets, which is applied in pharmaceutical formulations, medical preparations containing active ingredients, drug delivery, etc., can solve the problems of sodium picosulfate instability and stimulating effects, and achieve the reduction of adverse reactions, Avoid the effects of gastrointestinal discomfort
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
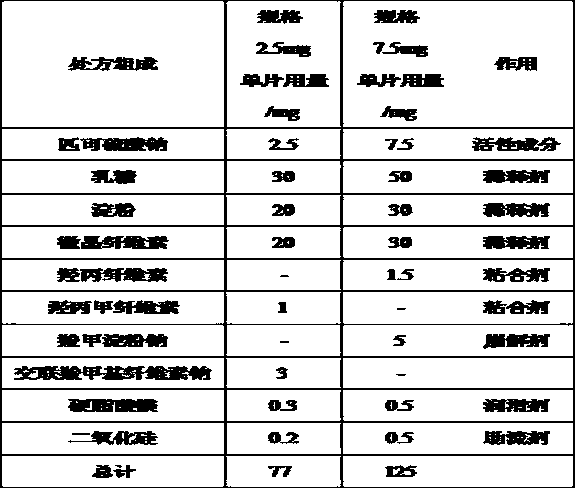
[0050] Example 1 Preparation of sodium picosulfate immediate-release tablet core
[0051]
[0052] Preparation method: ①Binder solution preparation: Weigh the prescription amount of hypromellose or hypromellose to prepare a 3% (w / w) aqueous solution. ②Material pretreatment: the particle size of sodium picosulfate is controlled below 80 mesh, and the particle size of other auxiliary materials is controlled below 60 mesh. ③ Mixing: Weigh the prescribed amount of sodium picosulfate, sodium starch glycolate or croscarmellose sodium, lactose, starch, and microcrystalline cellulose into a mixed wet granulator and mix well. ④ Granulation: Add the binder solution to the above materials, stir and granulate. ⑤Drying and granulating: drying at 45~55℃ and then granulating. ⑥Total mixing and tableting: Add magnesium stearate and silicon dioxide to the dry granules, then mix and tablet them for later use.
Embodiment 2
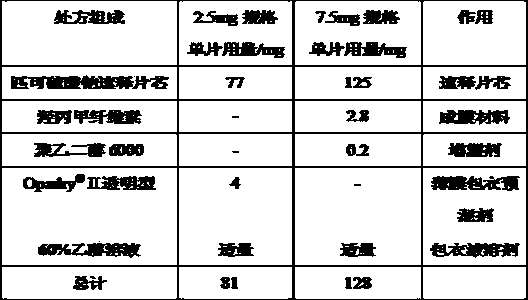
[0053] Example 2 Preparation of isolation layer of sodium picosulfate tablets
[0054]
[0055] Preparation method: ①Weigh hypromellose, polyethylene glycol 6000 or commercial film coating premix Opadry according to the prescription ? Ⅱ Dissolve in an appropriate amount of ethanol solution to make a coating solution, with a solid content of about 15%. ②The sodium picosulfate immediate-release tablet core is coated in a high-efficiency coating machine. The film-forming temperature is about 40~50℃, and the weight gain of the isolation layer is about 2~5% of the total weight of the immediate-release tablet core.
Embodiment 3
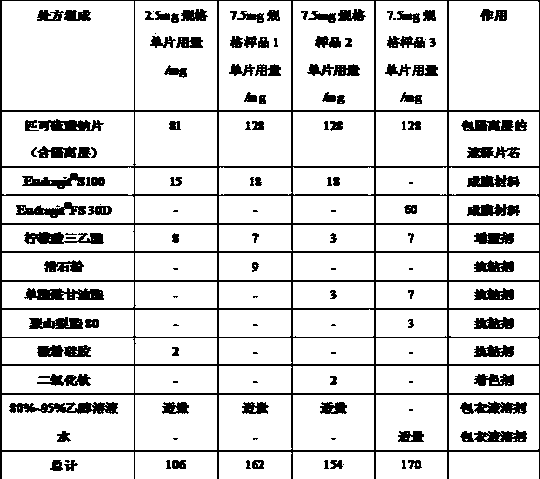
[0056] Example 3 Preparation of enteric coating layer of sodium picosulfate tablets
[0057]
[0058] Note: Eudragit ? FS 30D is a 30% water dispersion containing dry powder of methacrylic acid / methyl acrylate / methyl methacrylate (1:1:1) copolymer.
[0059] Preparation method: ①Weigh methacrylic acid / methyl methacrylate (1:2) copolymer according to the prescription [Eudragit ? S100] or methacrylic acid / methyl acrylate / methyl methacrylate (1:1:1) copolymer [Eudragit ? FS 30D], triethyl citrate, talc or micro-powder silica gel or monostearoyl glyceride and polysorbate 80, titanium dioxide are dispersed in an appropriate amount of ethanol solution or water to prepare a coating liquid, the solid content is about 15%. ② Put the sodium picosulfate immediate-release tablet core coated with the isolation layer into a high-efficiency coating machine, and the film-forming temperature is about 30℃. The weight gain of the enteric coating layer is about the total of the immediate-release tablet ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com